INTRODUCTION
An ageing society is characterised by the elderly population, classified as individuals aged 65 years or older, constituting ≥7% of the total population. Furthermore, when the ageing rate reaches 14%, the designation shifts to an aged society, and upon reaching 21%, it is termed a super-aged society. Japan achieved the status of an ageing society in 1970, transitioned to an aged society in 1994, and attained super-aged society status in 2007, marking it as the country with the world’s most elderly population. The speed of ageing is typically measured by the number of years it takes for the proportion of elderly individuals to increase from 7% (ageing society) to 14% (aged society). Remarkably, Japan transitioned to an aged society within a brief period of 24 years. It took France 126 years, Sweden 85 years, the USA 72 years, the UK 46 years, and Germany 40 years to undergo a similar transition. As such, Japan’s ageing population is progressing at an unprecedented rate.1 As of 2020, the percentage of Japanese citizens aged ≥65 years has exceeded 29%, and it is anticipated to reach 35% in less than 20 years.
This has led to an ageing population, not only among healthy individuals, but also among patients with rheumatoid arthritis (RA). The ageing of the population is not exclusive to Japan, and there is a global increase in the number of elderly individuals with RA. This rise is attributed not only to the ageing of the overall population but also to an increase in carryover, as advancements in treatment enable adolescent and middle-aged-onset patients with RA to survive longer. The incidence of elderly onset RA is also on the rise, although a definitive explanation has not yet been provided.
Several therapeutic challenges have been recognised in the treatment of RA in elderly patients, with the most significant being the compromised ability to metabolise drugs due to diminished organ function.2,3 Specifically, methotrexate (MTX), located in Phase I of the European Alliance of Associations for Rheumatology (EULAR) treatment recommendation for RA, relies on kidney-dependent metabolism. The decline in renal function associated with ageing elevates MTX concentrations, thereby heightening the risk of concentration-dependent adverse events, such as myelosuppression. Additionally, a decline of cognitive function and depressive tendencies pose a risk of reduced medication adherence and overdose. Furthermore, musculoskeletal deterioration (sarcopenia), compounded by impairments in activities of daily living due to the disease activity of RA itself, heightens the risk of becoming bedridden.4 Consequently, choosing drugs solely based on efficacy in the elderly population may lead to lower continuation rates due to their elevated susceptibility to infections. The treatment of elderly patients with RA is one of the most important issues because achieving remission or low disease activity, as recommended by EULAR, remains the goal even in older patients.5 Addressing this concern is crucial for enhancing the healthy life expectancy of elderly individuals and can contribute to mitigating the decline in the labour force and escalating healthcare costs.
As mentioned earlier, the World Health Organization (WHO) defines the elderly as individuals aged 65 years or older. However, recent data on the physical and mental ageing phenomenon among the elderly in Japan indicate that the majority of individuals aged 65–74 years maintain good physical and mental health, enabling them to engage in active social activities. In other words, the conventional inclination to categorise individuals aged ≥65 years uniformly as elderly is no longer realistic given the current scenario, as those aged 65–74 years significantly differ from those aged ≥75. Consequently, the treatment of patients with RA ≥65 years old and those ≥75 years old should be considered separately.
ACCUMULATION OF REAL-WORLD CLINICAL EVIDENCE (FIRST REGISTRY)
Since the approval of biologics for RA treatment in Japan in 2003, patients initiating molecular targeted therapy from the University of Occupational and Environmental Health, Kitakyushu, Japan, and neighbouring hospitals, have been included in a registry (Figure 1A and B).
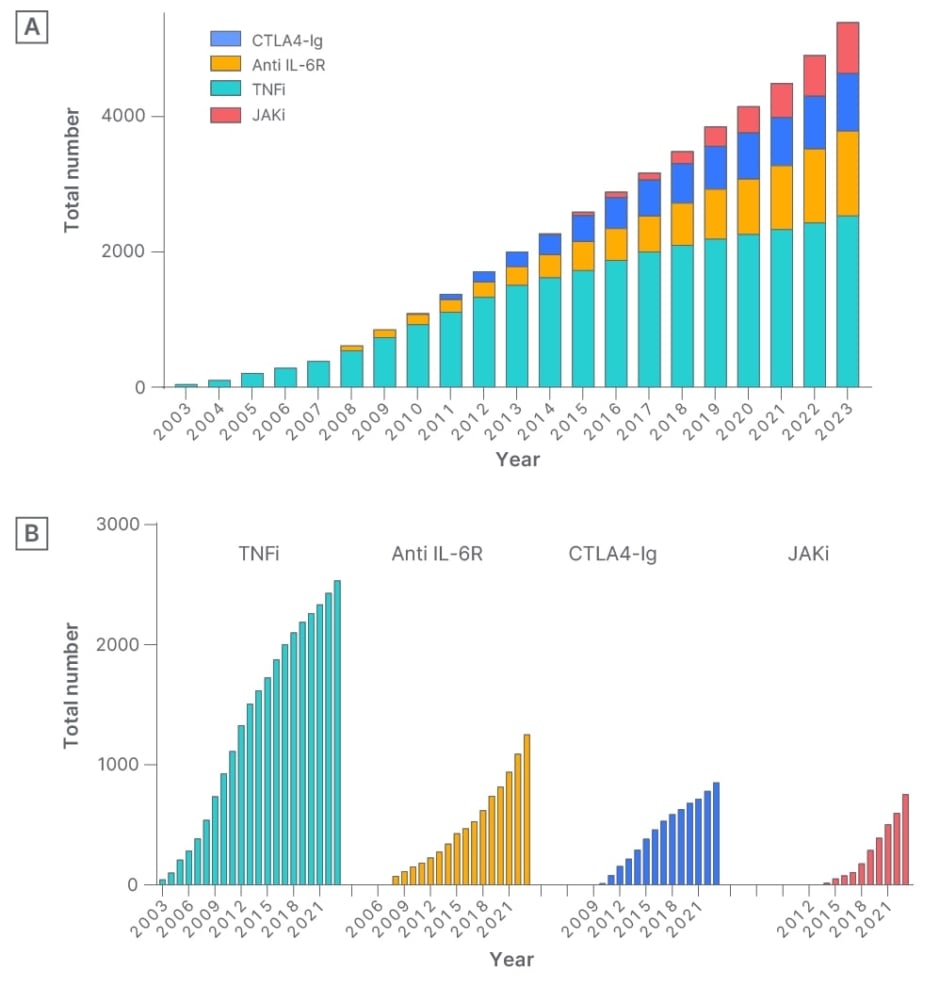
Figure 1: A) Cumulative use of molecular targeted therapies over the years: total number of all molecular targeted therapies. B) Cumulative use of molecular targeted therapies over the years: cumulative usage of each molecular targeted therapy, including TNF inhibitors, anti-IL-6 receptor antibodies, cytotoxic T lymphocyte-associated antigen 4-Ig, and JAK inhibitors.
CTLA4-Ig: cytotoxic T lymphocyte-associated antigen 4-Ig; JAKi: JAK inhibitors; TNFi: TNF inhibitors.
Short-term hospitalisation, which includes risk assessment and self-injection instruction following a clinical path, was implemented, and a systematic database is being constructed with the support of five data management staff. Presently, the registry includes more than 5,000 patients with RA, and real clinical data have been reported utilising this dataset. For instance, in a study on the withdrawal of biologic drugs in patients with long disease duration, deep remission was identified as a factor contributing to successful withdrawal of TNF inhibitors.6 Additionally, as a strategy to minimise selection bias inherent in real-world clinical data, the team have pioneered the use of propensity score matching and propensity score-based inverse probability of treatment weighting in the field of rheumatology. These methods effectively mitigated bias, facilitating comparisons of efficacy among drugs that had not undergone clinical trials.7,8
Real-world clinical data exhibit a lower level of clinical evidence compared to clinical trials. This is attributed to the inherent bias present in real-world clinical settings and the inability to predetermine study designs. However, patients are sometimes excluded from clinical trials due to age limitations or comorbidities. Specifically, elderly patients with RA frequently face exclusion from clinical trials based on these criteria, leading to a current lack of evidence regarding efficacy and safety for this group. Compared to Japan, where the population is ageing at an accelerated rate, the ageing of the population in Western countries is relatively mild. Nevertheless, addressing the treatment of RA in elderly patients aged >75 years is a challenge that Western countries also encounter. Japan, with its super-aged society, could offer valuable insights. Thus, the team investigated the persistence rates of molecular targeted therapies in the elderly demographic and conducted a detailed analysis of trends associated with specific drugs.9
OPTIMAL DRUG SELECTION FOR GERIATRIC PATIENTS WITH RHEUMATOID ARTHRITIS
Biologics theoretically selectively suppress signals related to their target molecules, and are generally unaffected by hepatic or renal dysfunction. Additionally, due to their favourable balances of efficacy and safety, it is considered an optimal treatment option for elderly patients. On the other hand, the findings from the ORAL Surveillance study on tofacitinib have raised concerns regarding the risk of malignancy and major adverse cardiovascular events.10 Consequently, both the U.S Food and Drug Administration (FDA) and European Medicines Agency (EMA) have issued warnings regarding the use of JAK inhibitors, particularly in patients aged >65 years.11 Given this context, the medical care of the elderly should aim to optimise the benefits of molecular targeted therapies and employ them judiciously. Hence, the team conducted a comparison of the 3-year retention rates for four classes of molecular targeted therapies (TNF inhibitors, anti-IL-6R antibodies, CTLA-4Ig, and JAK inhibitors) across three age groups (<65 years, 65–75 years, and >75 years) to ascertain their suitable utilisation for each demographic.9
Before the commencement of molecular targeted therapy, numerous variables, including erythrocyte sedimentation rate and the presence of comorbid hypertension, exhibited variations across age groups. Among these variables, the rate or quantity of concomitant use of MTX exhibited considerable variability (Figure 2). Ageing is the most significant risk factor for the decline in renal function, as it results in a reduction in glomerular count. Approximately 40% of individuals >65 experience a decline in estimated glomerular filtration rate to 60 mL/min/1.73 m2. The ageing process is associated with a diminished immune response, heightening the risk of infections. Additionally, there is a decline in pulmonary and cardiac reserve, amplifying the severity of adverse events. Moreover, the presence of coexisting lung disease, posing a risk for bacterial pneumonia development, was observed in 39.2% of individuals >75 years of age, contrasting with a rate of only 19.4% in those under 65 years; a notable two-fold difference. Indeed, a Japanese survey examining MTX-related deaths in patients with RA unveiled that 64.6% of the 851 reported deaths occurred in individuals aged ≥70 years. This underscores the heightened susceptibility of elderly patients to adverse drug reactions, likely influenced by diverse factors. In this context, the challenge of being unable to use MTX in Phase I or to combine MTX with molecular targeted therapy in Phase II and subsequent phases may pose a significant obstacle in the treatment of elderly patients with RA.
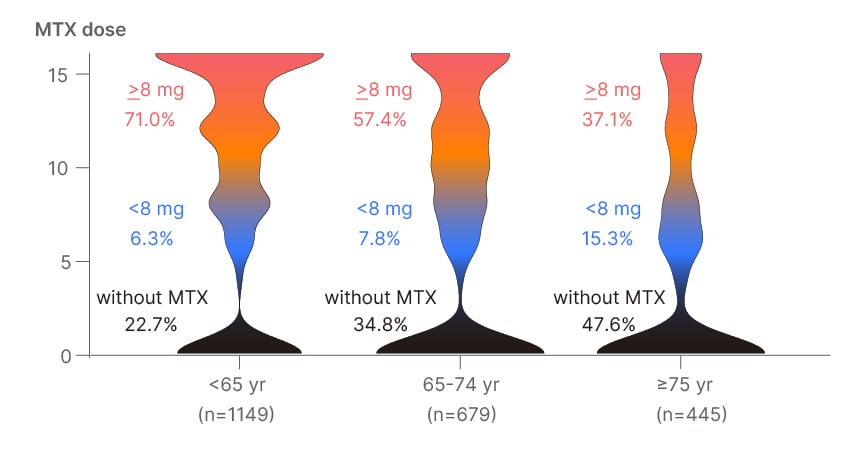
Figure 2: Methotrexate dosage per week across generations.
MTX: methotrexate; yr: years.
When comparing the continuation rates of molecular targeted therapies, JAK inhibitors and anti-IL-6R antibodies exhibited the highest rates of continuation among younger patients.9 In this study, the incidence of infection and its associated discontinuation at a young age was <1.0 per 100 patient-years for all drugs, suggesting that all drugs are relatively safe to use. Generally, younger individuals tend to have higher social activity needs, necessitating more robust suppression of disease activity. Therefore, considering their efficacy, JAK inhibitors were deemed more suitable for younger patients. Meanwhile, the retention rates of JAK inhibitors and abatacept were higher in the early elderly population, aged 65–74 years. It is noteworthy that drugs with distinct characteristics, such as the highly effective JAK inhibitor and the safer abatacept, exhibited the highest continuation rates. In this generation, TNF inhibitors and cytotoxic T lymphocyte-associated antigen 4-Ig were the only ones with infections and associated discontinuations <1.0 per 100 patient-years, while JAK inhibitors and anti-IL-6R antibodies showed an increasing trend. The diverse treatment goals for 65–74-year-olds emphasise the importance of shared decision-making. The persistence rates in the elderly (≥75 years) were highest for abatacept and anti-IL-6R antibodies. The incidence of infections and associated discontinuations in this generation was high for JAK inhibitors, exceeding 4.0 per 100 patient-years. This suggests that the use of JAK inhibitors in patients ≥75 years old should be considered with caution regarding the development of infections. On the other hand, the safety profile of abatacept was outstanding among molecular targeted drugs, with infections and associated discontinuations below 1.0 per 100 patient-years even among patients aged ≥75 years.
These findings highlight a crucial aspect: the reasons for discontinuation varied significantly across different age groups. In younger patients, discontinuations due to adverse events were less frequent, necessitating a more efficacy-based selection. Conversely, the incidence of discontinuations due to adverse events increased with older age. Particularly noteworthy are the variations of each molecular targeted drug in continuation rates observed among the early elderly, aged 65–74 years, as mentioned earlier. A unique follow-up study in Denmark has demonstrated that the younger a person appears cognitively (cognitive age), the more their physical function is retained, leading to an extension of life expectancy.12 Individuals aged 65–74 years exhibit diverse variations in physical function, encompassing factors such as heart and kidney function, and maintaining a youthful appearance may also play a crucial role. The observation that drugs with higher continuation rates differed between individuals aged 65–74 years and those aged ≥75 years is also significant for future clinical studies, and these two age groups should be considered separately.
However, the findings come with certain limitations. First, the evidence level is lower as the results were not based on a randomised prospective trial, posing potential challenges in generalising the results. Rituximab, known for its safety, was not included in the authors’ study, as it is not approved for RA in Japan. Additionally, the number of patients may be insufficient to accurately detect the rate of adverse events, requiring more power for such assessments.
CONCLUSION
Biologics theoretically do not inhibit any molecules other than their intended targets. Their metabolism is predominantly taken up and degraded by cells of the reticular system, akin to gamma globulin. Hence, serum concentrations of biologics are unaffected by renal or hepatic function. In this context, the successful utilisation of biologics, particularly in the elderly, is regarded as a crucial therapeutic strategy in the management of RA. The findings represent crucial evidence that enhances the basis for collaborative decision-making in clinical contexts. The team aspire for this research conducted in Japan, home to the world’s most aged population, to serve as a guiding framework for the global surge in ageing and the corresponding management of RA in the elderly.







