INTRODUCTION
Fibromyalgia (FM) is characterised by chronic musculoskeletal pain, autonomic nervous system (ANS) dysfunction, and disturbed sleep. In 2010, the American College of Rheumatology (ACR) included a patient-reported measure of unrefreshing sleep in its preliminary diagnostic criteria for FM and measurement of symptom severity.1
The ANS plays an important role in co-ordinating many bodily functions during sleep. Patients with untreated sleep disorders often describe symptoms of ANS impairment, such as morning stiffness, fatigue, depression, non-restorative sleep, and reduced cognitive performance,2 and the majority of patients with autonomic impairment have some form of sleep disorder.3 As the cause of sleep disorders in FM patients is still unclear, the aim of this study was to evaluate the influence of ANS dysfunction on the genesis of their sleep disorders.
METHODS
We enrolled 50 female FM patients and 45 female healthy subjects matched by age, sex, and BMI. All of the subjects underwent an 18 tender point examination1 and described the intensity of their somatic pain using a 100 mm visual analogue scale, before being administered a sleep questionnaire (to evaluate sleeping complaints)4 and the Epworth Sleepiness Scale.5 The sleep profile was evaluated by using a polysomnography comprising an electroencephalogram, a 6-channel electrocardiogram, and an electro-oculogram. Submental right and left tibialis anterior muscle electromyograms were recorded using surface electrodes and standard techniques.6 The respiratory disturbance index, the desaturation event frequency, periodic breathing (PB), and arousals index were scored in accordance with the criteria of the American Sleep Disorders Association (ASDA).6 The cyclic alternating pattern (CAP) is a marker of sleep instability composed of a Phase A (lumps of sleep phasic events) followed by a Phase B (return to electroencephalography background) and was identified using the scoring rules.7 CAP sequences include at least two consecutive CAP cycles.
The autonomic profile was evaluated at rest and during a tilt test to determine muscle sympathetic nerve activity (MSNA) using the microneurography technique,8 plasma catecholamine levels, and the spectral indices of cardiac sympathetic (low frequency component of RR variability [LFRR]) and vagal (high frequency component of RR variability [HFRR]) modulation computed by spectrum analysis of RR during sleep.
RESULTS
The FM patients had a higher heart rate (HR), more MSNA, a higher LF/HF ratio, and lower HFRR values at rest (p<0.05), showing no increase in MSNA, a smaller decrease in HFRR, and an excessive rate of syncope (46%) during the tilt test. Their sleep was less efficient (p<0.01), and they had a higher proportion of Stage 1 non-rapid eye movement (REM) sleep (p<0.001), experienced many arousals and periodic limb movements (PLM) per hour of sleep (p<0.001), and a high proportion of PB (p<0.0001). Their CAP rate was significantly increased (p<0.001) (Table 1). During sleep, they had a higher HR and LF/HF ratio, and a lower HFRR (p<0.001) (Table 2). The number of tender points, CAP rate, PB, and PLM index correlated positively with HR and the LF/HF ratio, and negatively with HFRR during sleep.
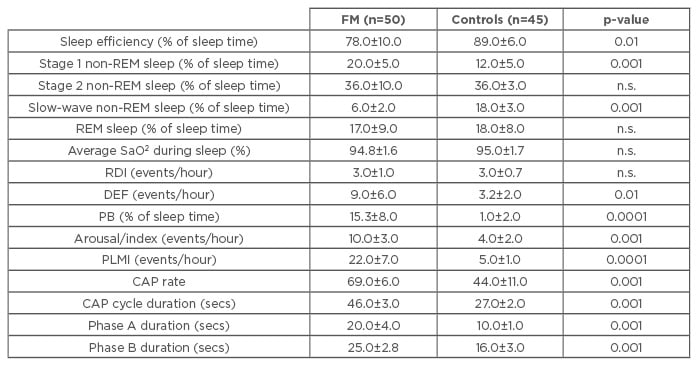
Table 1: Sleep parameters in fibromyalgia patients and healthy controls.
Phases A and B are components of the CAP. Variations during CAP involve different degrees of muscle tone, heart rate, and respiratory activity, which increase during Phase A and decrease during Phase B.
Data expressed as mean±standard deviation; significance threshold p<0.05.
DEF: desaturation event frequency; PB: periodic breathing; PLMI: periodic limb movement index; REM: rapid eye movement; RDI: respiratory disturbance index; SaO2: oxygen saturation; CAP: cyclic alternating pattern. FM: fibromyalgia; n.s.: not significant.
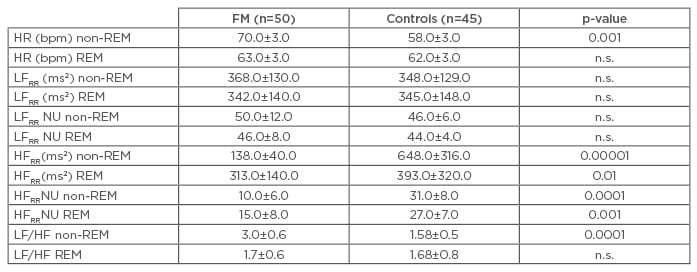
Table 2: Autonomic nerve system parameters variations during non-rapid eye movement (REM) and REM sleep in fibromyalgia patients and in controls.
Data expressed as mean±standard deviation; significance threshold p<0.05.
HFRR: high frequency component of RR variability; HR: heart rate; bpm: beats per minute; LFRR: low frequency component of RR variability; NU: normalised units; FM: fibromyalgia; ms2: metre per second squared; n.s.: not significant.
CONCLUSION
These data confirm that the FM patients have an ANS dysfunction that is consistent with sympathetic over-activity due to the intensity of chronic pain when awake and during sleep. These findings explain the excessive rate of syncope observed in the FM population during wakefulness and the increased presence of CAP, PB, and PLM during sleep.
As PB increases baroceptor gain and sympathetic outflow, it creates a vicious circle: the painful exacerbation increases sympathetic cardiovascular activation and reduces sleep efficiency, thus increasing light sleep, CAP rate, arousals, PLM, and the occurrence of PB, which in turn causes abnormalities in cardiovascular neural control and exaggerated pain sensitivity.








