Meeting Summary
Prof Welte opened the symposium by describing the key roles of hypercoagulation and inflammation in the course of severe coronavirus disease (COVID-19). This was complemented by Dr Larbig’s talk that introduced CSL312, a new human monoclonal antibody targeting coagulation factor XIIa, which aims to target both hypercoagulation and inflammation. Prof Idzko followed with a discussion about the challenges of treating respiratory conditions such as alpha-1 antitrypsin deficiency (AATD) during the COVID-19 pandemic. Dr Sucena explained that many patients had been unable or unwilling to attend health centres to receive alpha-1 antitrypsin (AAT) therapy, putting them at risk of increased morbidity. In response, Prof Herth discussed the use of self-administered AAT to ensure that patients receive regular therapy. Prof Greulich described the difficulties in providing robust evidence for the positive impact of AAT therapy on mortality, and Prof Sandhaus introduced a recent observational study that overcomes some of these problems, with findings that suggest AAT therapy results in improved survival rates and a slower decline in quality of life (QoL). Finally, Profs Idzko, Singh, and Chalmers emphasised that better treatments are needed for other respiratory conditions, and introduced drugs that are currently under development to address this need; these include a new antibody intended to treat a broad range of the severe asthma population (CSL311; CSL Behring, King of Prussia, Pennsylvania, USA) and nebulised IgG to treat non-cystic fibrosis bronchiectasis (NCFB) (CSL787; CSL Behring). Overall, advances continue to be made in the treatment of severe respiratory conditions, despite the difficulties posed by the current COVID-19 pandemic.
Challenges of Treating Lung Disease Caused by COVID-19
Professor Tobias Welte and Doctor Michael Larbig
Prof Welte described COVID-19, caused by the new coronavirus strain severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), as a disease that primarily affects the respiratory epithelium. The virus enters the bloodstream at an early stage and the course of COVID-19 is then dictated by damage to the vascular endothelium and vascular leakage.1 This means that unlike classic acute respiratory distress syndrome, severe COVID-19 is more likely in patients with endothelial dysfunction, such as those with diabetes, obesity, or arterial hypertension, compared with patients with respiratory diseases in general.1
Repair mechanisms in the vascular endothelium can lead to hypercoagulation that may cause thrombosis, leading to pulmonary emboli and affecting gas exchange. Recent research has shown that capillary thrombosis is a major pathomechanism of severe COVID-19 disease at the microvascular level.2,3
Drugs currently under investigation for COVID-19 treatment fall into three groups:
- Antivirals, such as remdesivir, aim to reduce the viral load, which may drive the early stage of COVID-19.
- Anti-inflammatories aim to reduce the hyperinflammatory burden, which is a consequence of the host immune response to endothelial damage.4 This immune response is driven by lymphocytes but also involves mediators such as cytokines.3-6 Patients with severe disease have been shown to have very high concentrations of cytokines, suggestive of a cytokine storm.
- Anticoagulants, such as heparin, aim to counteract the coagulation recently shown to be involved in COVID-19 disease.6-8 It remains to be seen whether anticoagulants with more specific targets than heparin could be used to reduce right–left shunting on the pulmonary level, which would reduce hypoxaemia and the need for mechanical ventilation in severe COVID-19.9 For example, CSL312 is a new drug that targets factor XIIa and is currently being tested in a Phase II clinical trial.10
Targeting Factor XII in COVID-19 Disease
Dr Larbig explained that factor XII becomes activated upon contact with damaged tissues, and in turn activates factor XIIa, which initiates the kallikrein–kinin system.11 This results in inflammation, vasodilation, and capillary leakage, which can cause fluid accumulation in lung tissue.11 Factor XIIa also triggers the complement system and the intrinsic coagulation cascade, which can lead to thrombosis (Figure 1).10,11 In addition, ex vivo human lung tissue models have shown that factor XIIa has proinflammatory properties.12
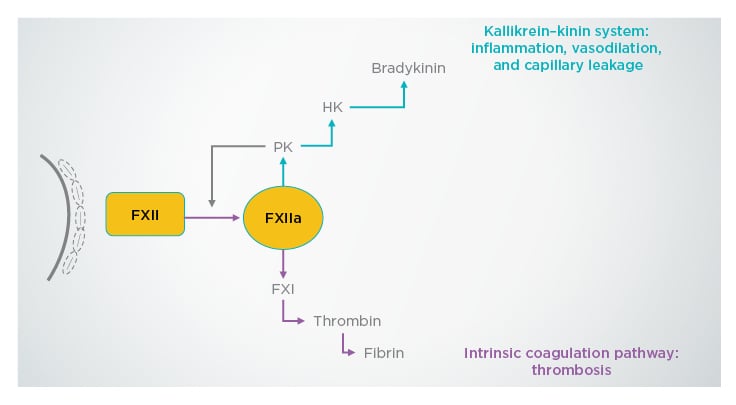
Figure 1: Mechanisms of action of factor XIIa on the immune system.
FXI: factor XI; FXII: factor XII; FXIIa: factor XIIa; HK: high molecular weight kininogen; PK: prekallikrein.
Adapted with permission from Weidmann et al.11
Growing evidence suggests that vascular leakage, pathological thrombosis, proinflammation, and complement activation may play a role in COVID-19,3,13,14 making factor XIIa a viable target for this disease. Human monoclonal antibody CSL312 has the potential to block the pathology triggered by activated factor XIIa, but to investigate whether it can influence disease progression in patients with severe COVID-19 disease, high-quality scientific data are needed. Despite the difficulties of conducting a controlled, randomised clinical trial during the current pandemic, investigators in many countries have shown that it is possible, and a placebo-controlled trial of CSL312 is currently underway in patients with COVID-19.10
Questions and Answers
Q: When do you expect to see preliminary results from the CSL312 trial targeting COVID-19 patients with severe pneumonia?
Prof Welte explained that many studies are currently underway to investigate potential treatments for COVID-19, but that few drugs have been found to be effective. He believes that addressing the coagulation pathway is one of the most promising ways to treat COVID-19 and emphasised that preliminary data from the CSL312 trial should be available in early 2021.
Q: What is the biggest challenge in treating COVID-19 patients with severe pneumonia?
Prof Welte replied that these patients have hypoxaemia and most require mechanical ventilation. Unlike other respiratory infections, patients with a severe course of COVID-19 need ventilation for weeks rather than days, and this increases the risk for cardiovascular and renal complications as well as secondary infections.
Q: Do you have any recommendations for protecting medical staff from COVID-19?
Prof Welte explained that in his hospital, double-masking was introduced from the beginning of the pandemic, preventing hospital-acquired infections. He recommends that in outpatient situations, social distancing, the use of face masks, and regular disinfection of the hands should be used to minimise transmission of the virus, as for any infectious disease.
Treatment of Alpha-1 Antitrypsin Deficiency in Times of COVID-19
Professor Marco Idzko, Professor Timm Greulich, Doctor Maria Sucena, and Professor Felix Herth
Prof Idzko highlighted that the treatment of severe respiratory diseases, such as AATD, is challenging during the COVID-19 pandemic. While there is a need to protect medical staff by introducing additional safety measures for diagnostic procedures, such as the lung function test,15 it is crucial to continue treating patients.
AATD is a genetic disease that results in reduced circulating levels of AAT (also known as α-1 protease inhibitor), a protein that protects lung tissue against the inflammatory enzyme neutrophil elastase.16 Patients with AATD are more susceptible to infection and structural damage of the lung, as well as liver disease caused by hepatocyte inclusions formed from misfolded AAT.16 Treatment options include regular AAT therapy to restore circulating levels of the protein.
Prof Greulich explained that although the risk of severe COVID-19 disease has not been assessed in patients with AATD, the fact that they are already severely ill means that they are more likely to have a poor outcome from COVID-19 than healthy patients. This makes it difficult to treat patients in many European countries because treatment is performed in health centres and attending these appointments will increase the risk of contracting SARS-CoV-2. In addition, the COVID-19 pandemic may restrict the resources available in these centres. If AAT therapy is interrupted, patients with AATD are at increased risk of lung infections, loss of lung density, and liver damage.16
Alpha-1 Antitrypsin Deficiency Treatment in Portugal During the COVID-19 Pandemic
Dr Sucena explained that the first case of SARS-CoV-2 infection in Portugal was confirmed on 2nd March 2020, and within a very short time frame, Portugal had to reorganise its national health service (Serviço Nacional de Saúde [SNS]) to focus on managing the COVID-19 pandemic. This meant that patients without COVID-19 had restricted access to hospitals and other health facilities: personal appointments were replaced with phone calls, day hospitals were closed or had their activities reduced, and bronchoscopies were only performed in urgent situations.
Although there were very few confirmed COVID-19 cases in patients with AATD in Portugal, Dr Sucena emphasised that the pandemic had a major impact on patients across the country because AAT treatment is only available in public day hospitals. During the first few weeks of the COVID-19 pandemic, AAT therapy was often cancelled, or patients were referred to alternative centres. Where treatment was still available, some patients chose not to attend because of the perceived risk of infection.
Health facilities in Portugal began to reopen to patients without COVID-19 in early May 2020, but as SARS-CoV-2 still exists in the general population, the SNS must evolve to manage COVID-19 while also caring for other patients. The course of the COVID-19 pandemic over the next few months will dictate the availability of AAT therapy to patients with AATD.
Prof Greulich highlighted that other countries are likely to face similar problems. The cancellation of appointments for patients with AATD will have left healthcare professionals unsure of the best way to care for their patients. Although some face-to-face appointments can be replaced by virtual appointments, other approaches are needed for treatment.
Alpha-1 Antitrypsin Deficiency Self-Administration in Germany and Austria During the COVID-19 Pandemic
A proportion of patients with chronic diseases have always been unwilling to attend appointments in health centres because of infection risk anxieties, and this problem has been exacerbated by the COVID-19 pandemic. However, for patients with AATD who are unable or unwilling to attend health centre appointments, a human AAT therapy (Respreeza®, CSL Behring) is licensed in Europe for at-home self-administration.17
Prof Herth emphasised the importance of selecting the right patients for self-administration of AAT therapy. Patients need to be physically and mentally fit, without significant comorbidities, and they should have the necessary motivation to learn how to self-administer their treatment. Prof Herth explained that in his preselected population, self-administration is suitable for 30–50% of patients with AATD. Many of these patients have been receiving intravenous AAT therapy for several years and are already familiar with the procedure. Training suitable patients is the next step, and guidance and support is offered for this process, including guidelines to help patients learn the required techniques (Figure 2).
Figure 2: Part of a step-by-step training guide for self-administration of alpha-1 antitrypsin therapy.
Prof Herth explained that the use of self-administered therapy by his patients guaranteed that they would receive their treatment on time every week, which has been extremely helpful during the COVID-19 pandemic.
Questions and Answers
Q: What is the legal situation for AAT therapy self-administration?
Prof Herth advised healthcare professionals to conduct well-documented, thorough training to protect staff legally.
Q: How many training sessions do you think are required for AAT therapy self-administration?
Prof Herth explained that this varied depending on the patient; for some, one training session might be enough, but generally about three sessions are required. If patients are still uncertain about the techniques, they can perform their initial infusions under the supervision of a healthcare practitioner in a medical centre.
Q: What, if any, adverse events might occur during AAT infusion?
AAT therapy has been used for many years, and for this reason, Prof Herth explained, physicians know that adverse events are unusual. Provided that patients have undergone adequate training, self-administration at home is as safe as administration in a health centre. Should an adverse event occur at home, the patient can stop the infusion.
The Impact of Alpha-1 Antitrypsin Deficiency Therapy on Mortality
Professor Timm Greulich, Professor Robert Sandhaus, Doctor Alice Turner, and Professor Tobias Welte
With many patients with AATD going untreated during the current pandemic, it is important to consider the impact that AAT therapy has on their overall mortality. Prof Greulich explained that an early study of AATD mortality using the National Heart, Lung, and Blood Institute (NHLBI) registry in the USA indicated that patients (N=1,129) who received intravenous AAT therapy had a lower risk of mortality compared with those who did not receive therapy (risk ratio: 0.64; 95% confidence interval: 0.43–0.94; p=0.02).18
More recently, an international, double-blind, randomised, placebo-controlled trial (RAPID) was conducted in 2015 to compare lung density in patients with AATD (N=180) who received AAT therapy with those treated with placebo.19 Over the 2-year study period, only four deaths were reported: one in the AAT group and three in the placebo group. The small number of patients in this study makes it difficult to derive important findings regarding mortality; a comparative study of mortality in patients with chronic obstructive pulmonary disease (COPD), for example, included >6,000 patients.20 To overcome this problem, more recent studies have used retrospective national registry data from around the world to investigate outcomes in patients receiving AAT therapy.
A Comparison of Outcomes Between AAT-Naïve and AAT-Treated Patients with AATD-Related Lung Disease Using Retrospective Data
Prof Sandhaus introduced a recent observational study that compared mortality, lung transplantation, and QoL data between matched patients with AATD-related lung disease in UK and USA national registries.21 Patients in the UK registry were treated with standard of care (control cohort), while those in the USA received standard of care plus plasma-derived intravenous AAT therapy (AAT cohort).21
Dr Turner explained that inclusion criteria included evidence of lung disease, PiZZ genotype (a homozygous substitution of lysine for glutamic acid at position 342) or worse AATD genotype, and age >18 years.21 Patients were matched by age, sex, baseline year, and smoking status.21 After matching, each cohort consisted of 655 patients, with a mean age of 52 years and a male predominance of approximately 60% in each group.21 Median follow-up time was 7 years in the control group, and 9 years in the AAT group.21 Approximately 6% of patients in each cohort received a lung transplant during the study period, and 193 of the control group died versus 167 of the AAT group (p=0.122).21
Kaplan–Meier analysis showed a statistically significant difference in overall survival between the two cohorts, with a 10-year survival probability of 68.5% in the control group and 80.0% in the AAT group (p<0.001).21 Among patients who received a lung transplant during the study period, those in the control group were likely to undergo surgery sooner, with a median time to lung transplant of 5 years, compared with 13 years in the AAT group (p<0.001).21 Because lung transplant is an important life event, Dr Turner stressed that delaying the need for surgery is a potential indicator of delayed disease progression.
Observational data has inherent limitations, such as confounding comorbidity and immortal time bias. For example, patients could have started AAT therapy many years before the AATD registry existed, and this treatment period would not be represented in the data. Respective analyses are being conducted on the data gathered for this study to address some of these issues.
One post hoc analysis that has already been performed is the assessment of QoL. Prof Sandhaus explained that any truly disease-modifying therapy might influence this outcome. By looking at QoL measured by the St. George’s Respiratory Questionnaire (SGRQ) over a specific time frame, immortal time bias can be eliminated, and to a large extent, one can control for the impact of factors like baseline forced expiratory volume in 1 second (FEV1) and comorbidities on this outcome. Upcoming data from this analysis indicate that QoL in patients who received AAT therapy declined more slowly than in controls.
Despite this study not fulfilling the criteria of a randomised clinical trial, it provides encouraging data on the positive impact of AAT therapy in patients with AATD. When the findings are translated to median life-years gained, an outcome commonly measured in clinical trials, patients receiving AAT therapy were found to gain around 6 life-years compared with controls. This is similar to data from a post hoc analysis of the RAPID trial, which indicate that AAT therapy may result in a gain of about 5.5 life-years, as measured by time to terminal respiratory failure estimated from the average group lung tissue atrophy.19
Questions and Answers
Q: What other investigations are planned to further investigate the impact of AATD treatment on mortality in the real world?
Prof Greulich described the European Alpha-1 Research Collaboration (EARCO),22,23 a new European Respiratory Society (ERS) Clinical Research Collaboration, which aims to collect international data from approximately 3,000 patients with AATD over the next few years. He explained that baseline data should be available in the near future, and longitudinal data will be available after 3–5 years, depending on funding. EARCO will provide the opportunity for researchers to match patients using a variety of baseline characteristics to minimise bias.
Development of Specialised Treatments for Severe Respiratory Conditions
Professor Marco Idzko, Professor Dave Singh, and Professor James Chalmers
While AAT therapy is effective at treating lung disease in patients with AATD, Prof Idzko emphasised that there remains an unmet need in other respiratory conditions, such as severe asthma and NCFB.
Targeting the Different Immunological Phenotypes of Severe Asthma
In the vast majority of patients with asthma, symptoms can be controlled with combination therapy of inhaled steroids and either long-acting muscarinic antagonists or long-acting β agonists.24 However, around 5–10% of patients have a severe form of asthma that cannot be controlled without maximal inhalation therapy.24 These patients experience considerable morbidity and account for approximately 50% of the total healthcare cost associated with asthma.25
Prof Idzko explained that progress has been made in identifying the immunological phenotypes of severe asthma over the past year. It is now possible to discriminate between allergic/nonallergic Th2 cell high (Th2-high) eosinophilic asthma, which is characterised by increased levels of eosinophils in the blood and sputum, but normal neutrophil counts, and Th2-low neutrophilic asthma, which is characterised by elevated levels of neutrophils.26
The improved understanding of immunological phenotypes has led to the development of new, targeted therapies for both allergic and nonallergic Th2-high eosinophilic asthma, with a particular focus on IL-5 pathways.26 However, Prof Idzko highlighted that these therapies are not always effective, and treatments for patients with Th2-low neutrophilic asthma have yet to be developed.
Prof Singh highlighted that granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, and IL-5 all play an important role in the maturation of a broad range of inflammatory cells, including eosinophils and neutrophils.27 All three cytokines are expressed at higher levels in the lungs of patients with asthma compared with controls. The fully human IgG4-κ monoclonal antibody (CSL311) is currently in drug development, which specifically targets the β common chain in the receptors for GM-CSF, IL-3, and IL-5, and could result in a broad range of anti-inflammatory activity.27,28 Compared with treatments currently used in clinical practice, CSL311 may be better placed to treat the broader severe asthma population, and a Phase I trial of CSL311 in patients with mild asthma is now underway, with results expected in 2021.28,29
Questions and Answers
Q: If we develop drugs that act on the broad range of severe asthma, will we still need to phenotype our patients?
Prof Idzko emphasised that although CSL311 could be effective in a broad severe asthma population, it is still important to look for phenotypic biomarkers such as eosinophils, IgE levels, and exhaled nitric oxide levels. Asthma therapy, both now and in the future, should always be personalised to the characteristics and phenotype of each patient.
Q: Some patients with severe asthma experience considerable limitations in their daily life. Could new treatments such as CSL311 improve QoL as well as exacerbation rates?
Prof Idzko explained that any treatment that reduces asthma exacerbations would be expected to improve QoL. However, measuring this outcome in clinical trial conditions will require long study periods with detailed questionnaires.
Reducing the Frequency of Exacerbations in Non-cystic Fibrosis Bronchiectasis
Prof Idzko highlighted that NCFB is another respiratory disorder that is challenging to treat. NCFB is defined as the irreversible, pathological dilation of the bronchi, and is characterised by recurrent infection, inflammation, persistent cough, and sputum production.30
Prof Chalmers explained that the therapeutic goal in NCFB is to prevent exacerbations, or to treat them early when they do occur to prevent further lung damage. Exacerbations are driven by bacterial infection, which also promotes neutrophilic inflammation that can lead to structural lung damage.30,31
In patients who experience frequent exacerbations, the ERS guidelines provide a conditional recommendation for the long-term use of antibiotics.32 However, there is limited evidence to support this approach,32 and the development of antibiotic resistance is a major problem. Prof Chalmers emphasised that there is a need for additional therapies, and one drug under investigation for NCFB is nebulised IgG (CSL787).
The proposed primary mode of action for CSL787 is prevention of exacerbations by reducing bacterial burden in the lungs. CSL787 may also enhance local immunity, with the potential to protect the lung from infection. Phase I studies are now being prepared to investigate the use of nebulised IgG to prevent chronic respiratory tract infection and the progression of lung disease.
Summary
Despite the challenges of providing routine healthcare and conducting clinical trials during the current COVID-19 pandemic, advances continue to be made in the development of drugs to treat severe respiratory conditions.