Meeting Summary
The vast array of inhaler devices can be overwhelming for patients with chronic obstructive pulmonary disease (COPD) or asthma. Matching the right inhaler features to patients’ needs is key to maximising adherence and achieving the best outcomes. During this symposium, leading global asthma and COPD experts took an in-depth look at the latest clinical data relating to inhaler satisfaction and clinical outcomes.
What Really Matters to Patients with Asthma and Chronic Obstructive Pulmonary Disease
Professor José Luis Izquierdo
The efficacy and safety of treatments for asthma and COPD are often the focus for healthcare professionals (HCP); however, there are other factors that should be considered to maximise patient adherence, such as the technical characteristics of inhaled therapy.1
Two systematic reviews of inhaler device use have shown, rather surprisingly, that a substantial proportion of HCP do not use inhalation devices correctly, and faulty inhaler technique by patients has not improved over a 40-year period (1975–2014).2,3Poor inhalation technique can significantly reduce the amount of drug deposited in the lung and thus reduce the effectiveness of treatment; therefore, correct inhalation technique is equally as important as the efficacy and safety of the pharmacological agent.
Importance of the Patient–Healthcare Professional Relationship
HCP require reliable information from patients in order to treat according to their needs.4In the COPD MIRROR study, most patients stated that they were not completely honest with their HCP, and a substantial proportion of pulmonologists and general practitioners did not recognise the insufficient frankness in their relationship with patients.5Improving the relationship between patients and HCP is important to allow open and honest discussion in order to make optimal decisions about relevant treatment options.
The main objective of COPD treatment from a patient’s perspective is to minimise symptoms, maintain daily activity, and avoid exacerbations;6,7however, HCP and patients do not always agree on the impact of disease on daily life or satisfaction with treatment. Discordance between patients and HCP has ultimately been shown to be higher in patients with poor asthma control compared with patients with controlled asthma.8
Patient Factors that Affect Adherence to Inhaled Therapy
Poor adherence to inhaled therapy and incorrect inhalation technique have an adverse effect on outcomes in asthma and COPD, even with the availability of effective treatment. For example, improper use of a pressurised metered-dose inhaler (pMDI) is associated with decreased asthma stability, and poor adherence to inhaled therapy is associated with increased mortality in COPD.9,10With this in mind, it is essential to
improve patient treatment satisfaction, because patients with high levels of overall inhaler satisfaction are more likely to be compliant.11
Several studies have evaluated patients’ inhaler preferences. For example, the ASCONA real-life study of 778 patients with moderate or severe asthma across 59 hospitals in Spain found that a higher proportion of patients reported satisfaction with dry-powder inhalers (DPI) compared with pMDI (52% versus 28%; p<0.001).12Furthermore, a higher proportion of patients were satisfied with Easyhaler®(Orion Corporation, Orion Pharma [Fin], Espoo, Finland) compared with Turbuhaler®(AstraZeneca UK Limited, Cambridge, UK), or Diskus®(GlaxoSmithKline, Brentford, UK) in a sub-analysis of 328 patients from the same study (62% versus 43%; p=0.01).13Ultimately, understanding patients’ inhaler preferences results in better adherence and asthma control.14
While HCP often focus on asthma and COPD treatments being effective and safe, patient preference for inhaler devices are also important as they affect adherence and outcomes.
Harnessing Inhaler Technology
Professor Federico Lavorini
The evolution of inhaled therapy for respiratory diseases dates back thousands of years. Important milestones in the development of modern-day inhaler devices for respiratory medicine include the introduction of pMDI and DPI in the 20thcentury.15
Technical Characteristics of Inhaler Devices
In pMDI, the drug and the propellant mixture are expelled from the metering chamber once the device is actuated. Although the appearance of modern-day pMDI has not changed significantly since the original device was introduced in 1956, there have been substantial improvements in the technical characteristics of the device, resulting in improved efficiency.16For example, innovations in aerosol formulations have led to smaller particle size, which increase lung deposition and decrease oropharyngeal deposition.17
In contrast to pMDI, DPI rely on patient’s inspiratory effort to generate the pressure drop which drives the airflow through the inhaler. The energy associated with the airflow is used to deagglomerate the small drug particles from larger carrier particles. As it is the patient’s inhalation which releases the powder, there is no requirement for hand-breath co-ordination. The turbulent airflow is produced by the patient’s inspiratory effort and the resistance of the inhaler.18The resistance to airflow through a DPI is a fixed property unique to each inhaler, while the inspiratory power is a property of the patient. Together these determine the airflow rate
through the inhaler.19
Optimal Drug Delivery with Inhaler Devices
There is a common misconception that patients with asthma or COPD may have difficulty
achieving sufficient flow through a high-resistance DPI. Peak inspiratory flow has historically been the key consideration for DPI; however, peak inspiratory flow alone cannot be used to compare flows between devices, and it should only be the focus when considering the flows through an individual DPI, as seen in Figure 1.18,20-22
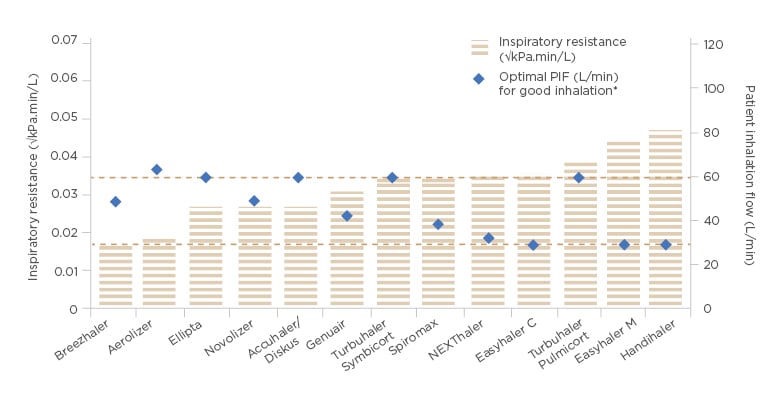
Figure 1: Inspiratory resistance and optimal peak inspiratory flow of marketed dry powder inhalers.
Accuhaler® (GlaxoSmithKline, Brentford, UK); Aerolizer® (Novartis, Basel, Switzerland); Breezhaler® (Novartis); Diskus® (GlaxoSmithKline); Easyhaler® (Orion Corporation, Orion Pharma [Fin] Espoo, Finland); Ellipta® (GlaxoSmithKline); Genuair® (AstraZeneca UK Limited, Cambridge, UK); Handihaler® (Boehringer Ingelheim Limited, Ingelheim am Rhein, Germany); NEXThaler® (Chiesi Limited, Parma, Italy); Novolizer® (Mylan N.V., Canonsburg, Pennsylvania, USA); Spiromax® (Teva Pharma B.V., Petah Tikva, Israel); Turbuhaler® (AstraZeneca UK Limited).
*Optimal PIF refers to the lower limit of desired inspiratory flow.
C: combination therapy; M: monotherapy; PIF: peak inspiratory flow. Adapted from Levy ML et al.22
The inspiratory power of the patient determines the amount of energy available for the powder deagglomeration. The same energy output can be achieved either with a low inhalation flow through a high-resistance inhaler, or a high inhalation flow through a low-resistance inhaler. For example, with the medium-to-high resistance device Easyhaler, it has been shown that patient inspiratory flow of 30 L/min is sufficient for powder deagglomeration and consistent dose delivery. Almost all patients with asthma and COPD have been shown to achieve this flow rate.21,23-26
Dose delivery, consistency, and robustness for daily use are important characteristics of inhaler devices. Anin vitrostudy comparing dose delivery of budesonide and formoterol with the Easyhaler and Turbuhaler at different patient airflow rates, showed that Easyhaler has superior dose delivery and consistency at all inhalation flows compared with the Turbuhaler (Figure 2).
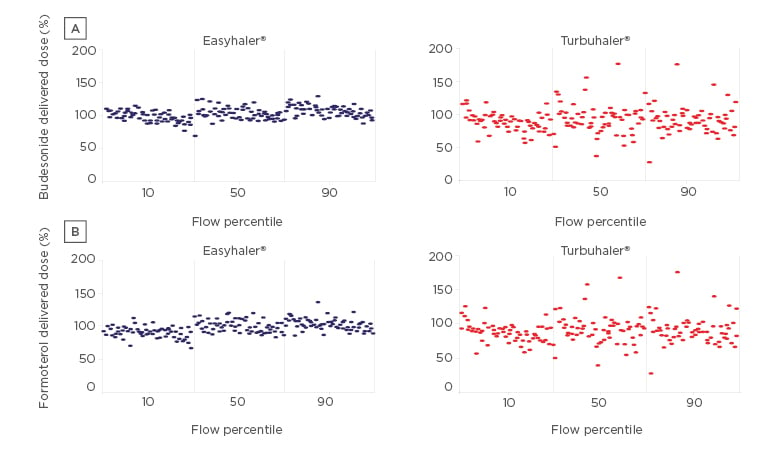
Figure 2: Dose delivery of (a) budesonide and (b) formoterol with the Easyhaler® and Turbuhaler® at different flow percentiles.
Easyhaler® (Orion Corporation, Orion Pharma [Fin] Espoo, Finland); Turbuhaler® (AstraZeneca UK Limited, Cambridge, UK). Adapted from Haikarainen J et al.24
Environmental variations in moisture, dropping the device, vibration, and freeze-thawing similarly does not affect consistency of dosing with the Easyhaler.24
Modern inhaler devices are the result of decades of research and innovative engineering. The inspiratory effort of the patient and resulting energy associated with the airflow are the key factors for deagglomeration and drug delivery, irrespective of the device resistance.
Patient Factors in Successful Asthma and Chronic Obstructive Pulmonary Disease Therapy
Doctor Mark L. Levy
Patient satisfaction and correct use of inhaler devices are integral factors in the management of asthma and COPD; however, inhaler technique errors are common and tend to increase with patient age and device complexity.27-29An association between inhaler technique errors and poor outcomes for patients with asthma or COPD has been published widely.30-33
Encouragingly, a study of >1,600 patients with asthma or COPD found that those who had more than one inhaler check had a lower risk of making critical errors, emphasising the positive impact of regularly checking inhaler technique.33Switching perspective to the use of inhaler devices by HCP, a study has shown that a large proportion of newly qualified clinicians were unable to correctly demonstrate inhaler devices.34Similarly, another study showed that only 5% of newly qualified medical interns used a pMDI perfectly; however, this increased to 73% after an intensive one-on-one training session.35
Appropriate Inhaler Selection
Six requirements for an ideal inhaler from a patient perspective have been summarised to help guide inhaler selection: effective, efficient, engaging, error-tolerant, easy-to-teach, and easy-to-switch to.22While inhaler satisfaction has been shown to increase adherence and asthma control,12patients will not always choose the inhaler that they are able to use correctly. A randomised, crossover comparison study of 50 patients with asthma or COPD found that while a higher proportion of patients were able to use Diskus with no critical errors compared with Turbuhaler (92% versus 74%; p=0.023), more patients expressed a preference for Turbuhaler than Diskus (25 versus 17 patients).36
The ASCONA real-life study confirmed that high patient satisfaction with an inhaler correlated
with better adherence and asthma control (Figure 3).12Switching inhaler device is also a strategy that can improve outcomes, as demonstrated by a Swedish study of 117 adult patients with asthma who were using budesonide–formoterol Turbuhaler and switched to treatment with budesonide–formoterol Easyhaler. After switching from Turbuhaler to Easyhaler, patients had equivalent or improved disease control and improved quality of life.37
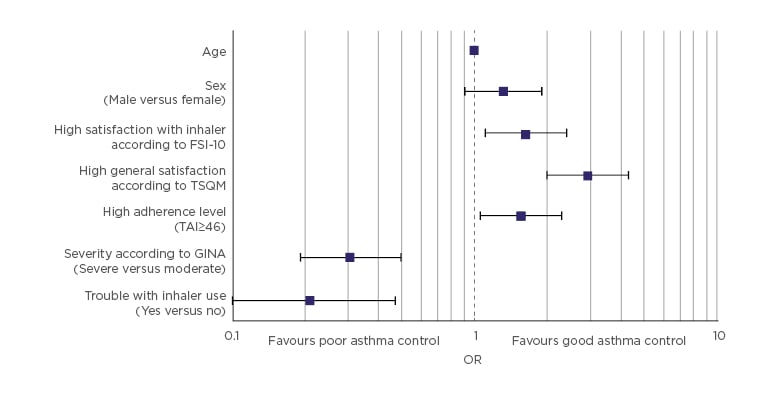
Figure 3: Asthma control according to the Asthma Control Test (ACT) score as the dependent variable.
ACT: Asthma Control Test; FSI-10: Feeling of Satisfaction with Inhaler questionnaire; GINA: Global Initiative for Asthma; OR: odds ratio; TAI: Test of Adherence to Inhalers; TSQM: Treatment Satisfaction Questionnaire for Medication.
Adapted from Plaza V et al.12
Education of Inhaler Technique
There are various methods to improve patients’ inhaler technique, including training devices, videos of inhaler technique, websites, pharmacist-led individual and small group education, and specialist paediatric technique clinics.38-41Repeated assessment and correction of inhaler technique is vital to sustain optimal delivery of medication to the lungs and improve health outcomes. For example, a randomised controlled trial showed that repeated instruction by trained pharmacists at 0, 1, 2, 3, and 6 months significantly improved inhaler technique and lung function. Correct inhaler technique notably decreased after 3 months, emphasising the need to check inhaler technique regularly.42
Correct training on inhaler device technique is not only important for patients, but for prescribers and pharmacists. It is important to ensure patients are satisfied with their inhaler and repeated inhaler technique education is provided, to ensure better adherence and outcomes.
Empowering Patients with Maintenance and Reliever Therapy
Professor Eric Bateman
There are several evidence-based methods that can improve treatment adherence in asthma. For example, clinical approaches such as home visits by trained asthma educators, and eHealth solutions, such as inhaler reminders sent to mobile phones;43,44however, the challenge with these strategies is sustaining enthusiasm to achieve long-term adherence and clinical benefit. Empowering patients with asthma to self-manage their disease using a therapeutic approach that harnesses their typical human behaviour has the potential to increase adherence and improve outcomes.
Two therapeutic strategies have been shown to improve adherence: use of once-daily rather than twice-daily therapy, and the maintenance and reliever therapy (MART) approach.45-48In MART, the combination inhaler containing a reliever (such as formoterol, a long-acting β2-agonist with rapid onset of bronchodilator action) and a low-dose inhaled corticosteroid (ICS) is used both as maintenance treatment and for relief of symptoms. MART is highly effective in reducing the frequency of worsening and severe exacerbations of asthma. This is attributable to the fact that the treatment is given early during asthma worsening, and effectively ‘titrated’ against symptoms, resulting in increasing administration of the ICS component when an exacerbation threatens. Patients on MART no longer require a short-acting β2-agonist (SABA).
A number of studies have confirmed that budesonide–formoterol MART reduces severe exacerbations by 30–70% compared with a high-dose ICS plus as-needed SABA and a long-acting β2-agonist/ICS combinations plus as-needed SABA at both the same dose of the former, and given at a higher dose.49-54A post hoc analysis of data from the AHEAD study demonstrated that the greater the need for reliever use (>6 or >8 inhalations/day versus >2 or >4 inhalations/day of reliever), the greater the benefit of budesonide–formoterol MART in terms of the risk of an exacerbation after the first high-use reliever day.54
In a study evaluating the efficacy and safety of a maintenance and reliever budesonide–formoterol combination inhaler in patients with asthma at risk of severe exacerbations, not only did MART reduce exacerbations compared with a standard fixed-dose regimen of budesonide–formoterol with as-needed salbutamol, but it reduced nonadherence to therapy.55
Over-Reliance on Reliever Therapy
With the aid of electronic monitoring of inhaler use, an exploratory post hoc analysis assessed inhaled β2-agonist use 14 days before patients presented to hospital with severe asthma. A large proportion of patients took very high doses of inhaled β2-agonists for prolonged periods, above the threshold that ought to have prompted medical review.56 Therefore, it is important for patients to seek medical attention instead of overusing a SABA to attempt to prevent potentially life-threatening events. A written action plan for patients to self-manage exacerbations can act as an ‘asthma safety net.’57Three evidence-based medication strategies include quadrupling maintenance dose of ICS, self-administration of high-dose oral corticosteroid, and MART or anti-inflammatory reliever approach.
Use of Maintenance and Reliever Therapy in Mild Asthma
The use of MART in mild asthma has also been investigated.58-60In the SYGMA-1 randomised, double-blind trial, the rate of severe and moderate exacerbations decreased by 60% and the rate of severe exacerbations by 64% with as-needed budesonide–formoterol compared with as-needed terbutaline in patients with mild asthma.58
In the NOVEL START real-life study of anti-inflammatory reliever use in mild asthma, the risk of asthma exacerbations was lower with as-needed budesonide–formoterol than with as-needed albuterol. This study also defined asthma worsening as a high-use episode, an urgent medical care consultation, or a prescription of systemic glucocorticosteroids. The number of times the criteria were met for urgent medical care was 36 in the albuterol group, 24 in the regular budesonide maintenance plus SABA group, and 13 in the budesonide–formoterol as-needed group, with a similar trend for a course of systemic glucocorticoids. Thus, budesonide–formoterol as-needed was shown to be superior to the current standard of care at Step 2 of the Global Initiative for Asthma (GINA)treatment steps.61
Taking these data into consideration, the 2019 Global Initiative for Asthma Global Strategy for Asthma Management and Prevention no longer supports SABA-only treatment for Step 1, recommending that a bronchodilator given for relief of symptoms should always be accompanied by low-dose ICS, preferably in one inhaler. As-needed formoterol combined with an ICS is presented as the preferred reliever across all steps of asthma treatment. This therapeutic strategy empowers patients to self-manage their asthma with one single inhaler and offers a continuity of care across treatment steps.
Summary
Professor Piotr Kuna
Patient inhaler satisfaction, preference, and correct technique are key to achieving better asthma control and improved COPD outcomes. With high resistance devices, the flow rate required to achieve same power output for powder deagglomeration is lower than for low resistance devices; thus, the inspiratory effort is rarely a limiting factor when choosing the inhaler. The simple one-inhaler MART approach matches typical human behaviour to empower patients to take control of their disease.








