Meeting Summary
Exacerbations in chronic obstructive pulmonary disease (COPD) impose a substantial healthcare burden and are key drivers of negative clinical outcomes and reduced patient quality of life. Prof Stockley highlighted the main differences in exacerbations between alpha-1 antitrypsin deficiency (AATD) and non-AATD-related COPD and considered potential implications for patient management. Early treatment of exacerbations with purulent sputum is known to be associated with improved patient outcomes. Emerging evidence from clinical studies also suggests that alpha-1 antitrypsin (AAT) therapy can have a positive impact on the nature and course of exacerbations in AATD.
Dr Zanichelli outlined how self-administration of intravenous drugs, which is a routine procedure that patients safely implement in other indications, has the potential to be successfully used by carefully selected AATD patients. Reflecting the current trend towards a more personalised approach to AATD therapy, self-administration can empower patients to assume an increasingly active role in their own disease management, thereby bringing improvements in treatment satisfaction, disease control, clinical outcomes, and quality of life.
A unique patient’s perspective on AAT self-administration was provided by Karen Skålvoll, who highlighted the key benefits offered by self-infusion, such as reduced localised trauma and increased freedom to travel and enjoy life. Photographs from Karen’s many global travels illustrate the unparalleled freedom that self-administration has afforded her as an AATD patient.
From the physician’s standpoint, Prof Sandhaus summarised his experience of how patients can be empowered to self-administer AAT therapy independently with minimal training. Among motivated individuals, self-administration can provide a successful long-term treatment solution for their AATD. The drive towards self-treatment also delivers the dual benefits of reduced healthcare burden and enhanced convenience and flexibility for patients.
Prof Koczulla reported that, overall, the available evidence indicates pulmonary rehabilitation as a successful strategy in AATD, which can significantly enhance a patients’ physical performance. Although the most effective training algorithm still needs to be prospectively validated, this approach may prove particularly advantageous in patients with anxiety, dyspnoea, and fear of physical activity. In order to achieve maximum benefit, therapy and goals of pulmonary rehabilitation must always be tailored to the individual patient in a personalised approach to care.
The meeting concluded with the compelling ‘AATD Strongman Contest,’ which pitted Prof Koczulla against AATD patient Karen Skålvoll in a physical test of endurance (the so called ‘farmer’s walk’ involving carrying a heavy obstacle) and strength (dumbbell raises). Notwithstanding the expected impairment in aerobic ability, the domination of Karen in the strength test clearly demonstrates the physical gains that patients with AATD can achieve with physical training.
Acute Exacerbations in Chronic Obstructive Pulmonary Disease: Are They Different in Alpha-1 Antitrypsin Deficiency?
Professor Robert A. Stockley
COPD is an umbrella term used to describe a number of progressive lung diseases, including emphysema and chronic bronchitis. AATD is a rare inherited condition caused by a deficiency in the enzyme inhibitor AAT, which predisposes individuals to both lung and liver diseases.1Therefore, patients with AATD lack the protective effect of AAT in the lung, rendering them more vulnerable to the damaging effects of smoking and/or infection.1
Both COPD and AATD are associated with acute events known as exacerbations where lung symptoms worsen suddenly, contributing to substantially poorer outcomes and reduced quality of life.2An exacerbation is characterised by worsening of the patient’s respiratory symptoms beyond normal day-to-day variation and leads to a change in medication.3
Different types of exacerbations are defined according to the Anthonisen criteria (Table 1).4
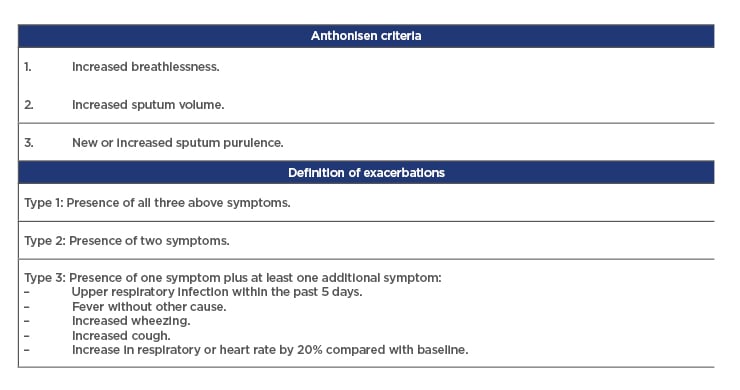
Table 1: Anthonisen criteria.
Evidence suggests that acute exacerbations in AATD are different to those experienced with usual (nondeficient) COPD in terms of both pathogenesis and severity. One study, examining the nature and effect of exacerbations in 265 patients with AATD, discovered that exacerbations occur commonly and are associated with declining health status.2 During the first year of study, exacerbations occurred in 142 subjects (54%), with 47 (18%) experiencing frequent (≥3) exacerbations. A clear relationship was seen between the number of exacerbations and the St George’s Respiratory Questionnaire total score, indicating worse health status with more frequent episodes. The median length of each exacerbation in the AATD study was 14 days. This compares to a shorter average exacerbation length of 7 days documented in patients with typical COPD.5In addition,exacerbations associated with purulent sputum were associated with significantly worse symptoms at presentation than those with mucoid sputum.6
Differences have also been noted between AATD and usual COPD exacerbations in sputum colour, and the concentration of neutrophil elastase and cytokines, even though the degree of bacterial colonisation/infection provides a similar initiating drive. Studying these differences provides important insights into the underlying disease processes in COPD versus AATD and how these contribute to the manifestation and severity of exacerbations and their consequences. AAT is a member of the serine protease inhibitor superfamily of proteins and its main function is to inhibit neutrophil elastase in the lung.1 Deficiency of AAT results in increased levels of uninhibited neutrophil elastase leading to breakdown of lung tissue and emphysema-like changes.1 Bacterial load is directly correlated with the same initiating IL-8 levels in both AATD and usual COPD. However, AATD has been shown to be associated with a greater neutrophil load, and higher elastase activity which drives leukotriene B4production, and epithelial damage leading to greater serum protein leak than in matched patients without the deficiency.7However, after treatment with antibiotics there was a reduction in sputum myeloperoxidase levels activity indicating a reduction in neutrophil recruitment and the other cytokines. Despite this, elastase activity persisted, as did the sputum chemoattractant leukotriene-B4, remaining higher than baseline data in the COPD cohort.8
The impact of these worse exacerbation manifestations in AATD may have important implications for clinical management and treatment decisions. Protease inhibitor Z is the most common deficiency allele in AATD, with a large majority of individuals with severe disease being homozygous for the deleterious allele (PI*ZZ).9 A prospective study looking at the natural history of AATD in 43 patients with the protease inhibitor Z phenotype over 2 years showed that annual decline in forced expiratory volume (FVC) was directly related to exacerbation frequency (r=0.50; p<0.001).10These results show that progression of lung function decline in AATD is exacerbations, underscoring the importance of prompt treatment for these episodes. Despite this, evidence suggests that delays or failure to treat exacerbations in AATD are common. In one study, delay was shown to be influenced by a number of factors including symptom score, severity at onset, airflow obstruction, and lung density.11 Treatment delay was shorter in patients with higher symptom scores at onset and in those with lower baseline FEV1, FEV1/ forced vital capacity, and 15thpercentile lung density. The key independent predictor of delay in starting treatment was lung density (which is a direct measure of the amount of emphysema), as demonstrated by multivariate analysis.11There also appeared to be a significant association between Anthonisen criteria and length of a treated exacerbation, with Type 1 events lasting longer than Type 2, which were in turn longer than Type 3. Resolution of exacerbation after treatment initiation was found to be unaffected by the delay in therapy, but was correlated negatively with gas transfer, a physiological measure of the emphysema.11
Overall, the substantially higher protease activity appears to be the main differentiator in the disease process between AATD and typical COPD and may be attributable to the worse exacerbations seen in the former group. This is of great importance because proteases such as neutrophil elastase drive proteolytic degradation of lung tissue in patients with AATD, which is allowed to continue unopposed due to already reduced levels of protective AAT.12 Prof Stockley stressed that new or a worsening of sputum purulence is mandatory for antibiotic therapy, as it indicates the presence of new or increasing bacterial growth. In addition, early initiation of treatment is an important step towards reducing total exacerbation length.
Self-Administration: Taking Patient Empowerment Seriously
Doctor Andrea Zanichelli
AAT replacement therapy is currently the only available treatment that targets the underlying cause of lung disease in AATD. Treatment requires lifelong weekly intravenous infusions of AAT therapy with the approved dose of 60 mg/kg, typically administered by a healthcare professional (HCP) in the clinic or physician’s office. The associated travel and waiting times impose a significant burden on the patients’ everyday lives and contribute to treatment dissatisfaction. As a result, there is an increasing interest in intravenous self-administration of AAT therapy as a means of optimising the treatment experience and potentially enhancing the patients’ quality of life. Self-administration has the scope to deliver a raft of benefits for individuals with AATD, including greater independence and autonomy, fewer healthcare visits and hospitalisations, and active involvement in their own care.
Self-treatment with intravenous drugs outside of the clinic is already commonplace in other rare inherited conditions such as hereditary angioedema(HAE)and haemophilia, and important lessons can be learnt from this experience to support the paradigm of self-administration in AATD.13-15 Central to the successful implementation of this approach are educational programmes designed to support the self-administration of therapy and overcome key issues, such as fear of injections and lack of skills or confidence.16-18 Furthermore, patients and their families must learn the technical skills needed for self-infusion and the associated practical issues.
Similar initiatives in HAE have proven successful, with studies demonstrating the feasibility and positive impact of self-administration training.16,19 SABHA was an observational, single-centre, prospective study that assessed the self-administration of plasma-derived nanofiltered C1 inhibitor (pnf C1-INH) in 15 patients with HAE.16It should be noted that the low patient number included in this study was due to the rarity of HAE as a disease. Patients, sometimes accompanied by caregivers, underwent a self-infusion training course prior to the study. This consisted of a theoretical session taught by a physician, followed by a nurse-led practice session on an artificial arm. Administration courses were then performed in small groups, consolidated by a second HCP-supervised practical session (if required) for those still lacking confidence. Overall, results from this study showed a trend towards improved quality of life in patients self-administering, together with significant growth in global satisfaction scores (p=0.0072) between the first and second quarter of drug use. Notably, the percentage of patients reporting high levels of stress decreased over time, demonstrating improved confidence with treatment and a greater ability in self-infusing.
Second-generation products are now available for AATD and offer the advantages of higher specific activity, easier reconstitution, and shorter infusion times, which increases their amenability for use in the home environment. Self-administration of the AAT therapy Respreeza® is currently approved in the European Union (EU) but is rarely used because access to treatment imposes a major limiting factor.20Although no guideline recommendations for self-administration in AATD currently exist, some patients are already successfully self-infusing. Dr Zanichelli concluded that moving forward, and with appropriate education and training, self-administration has the potential to be more widely implemented in carefully selected patients with AATD. This is an important step on the road to personalised therapy, freeing patients from their current treatment burden and empowering them to seize greater control of their own disease and its management.
A Patient’s Perspective on Alpha-1 Antitrypsin Self-Administration
Karen Skålvoll
Ms Skålvoll, a patient with AATD and lung disease, described her own personal experiences of intravenous self-infusion, highlighting the increased independence and greater control that patients can gain by adopting a central role in their own treatment. Although patients may be unaware or nervous about self-administration, it is important to communicate the potential benefits that can be attained, such as reduced localised trauma at the infusion site, and explain how readily and safely the technique can be learned and performed. Access to home treatment offers patients greater freedom from the shackles of hospitalisation, providing practical advantages like flexibility and convenience together with wider benefits such as an increasing sense of autonomy and dignity in the AATD treatment process.
In an insightful video set in her own home, Karen demonstrated how easily the practicalities of reconstitution and intravenous infusion of AAT therapy can be carried out. Photographs from Karen’s many global travels highlight the unparalleled freedom and enhanced enjoyment of life that self-administration has afforded her.
Figure 2: A holistic approach to treatment.
A Physician’s Perspective on Alpha-1 Antitrypsin Self-Administration
Professor Robert A Sandhaus
Prof Sandhaus explained that selecting the right patients for self-administration of AAT therapy is pivotal, as the patient’s own capacity to self-administer effectively is likely to be the critical factor in success. Key considerations for the implementation of self-administration include the patient’s age, dexterity, motivation, disease status, and cognition.
A questionnaire sent to AATD experts indicated that they see an impaired ability to monitor patient adherence and potential safety issues surrounding intravenous infusion as the main concerns to self-administration.18 However, data from a recent AlphaNet21survey of patients included in the Alpha-1 Disease Management and Prevention Program (ADMPP) showed that a number of patients are already successfully and safely self-administering their AAT therapy.17 This cross-sectional, observational, telephone survey included patients aged ≥18 years diagnosed with AATD and receiving AAT therapy with Respreeza®.The overarching aim of the survey was to assess the occurrence, practicalities, satisfaction, and challenges of AAT self-administration. Of the 555 patients surveyed, only a minority (7.9%; 44 patients) were found to be actively self-administering their AAT therapy. However, all patients self-administering described being either ‘very satisfied’ (95.4%) or ‘satisfied’ (4.6%) with their treatment. The survey also revealed that patients who were self-administering required minimal training and infusions did not appear to differ from those conducted by HCP. A slight majority of patients (56.4%) required only 2–3 training sessions to learn how to successfully self-administer, with this training largely provided by a home nursing agency. Infusion duration was generally aligned with licensing recommendations and administration in the hospital-setting, with most patients requiring <1 hour to carry out the infusion. Encouragingly, patients experienced few problems with self-administration, with the majority (83.7%) reporting no difficulties whatsoever; only five respondents described infusion-related issues at some point in time. Almost all patients (~98.0%) also confirmed that easy access to help or advice was available if required.
In conclusion, Prof Sandhaus noted that the overall results of the AlphaNet survey should help to address and assuage physician concerns by demonstrating that self-administration can, and is, being safely performed in patients with AATD. Among motivated individuals, self-administration can prove a viable long-term treatment approach, with nearly 20% of the surveyed patients successfully self-infusing their AAT therapy for over a decade. Careful selection of patients is essential to optimise the benefits of AAT self-administration, and appropriate physician-led follow-up is also important, providing help and advice with infusion-related issues where required.
What Pulmonary Rehab Can Do for Alpha-1 Antitrypsin Deficiency Patients
Professor Andreas Rembert Koczulla
Pulmonary rehabilitation is a comprehensive intervention in lung disease that is based on thorough patient assessment followed by patient-tailored therapies, including, but not limited to, exercise training, education, and behavioural change. These are designed to improve the physical and psychological condition of people with chronic respiratory diseases and promote the long-term adherence to health-enhancing behaviours.22Although rehabilitation is currently recommended for AATD-related COPD, there is no reference to AATD-specific studies in the guidelines and there is scarce evidence on the impact of training in this particular patient cohort.22
Prof Koczulla cautioned that patients with ATTD have very individualised problems that may affect their pulmonary rehabilitation, most notably physical and psychological comorbidities. These treatable traits, which include anxiety or depression, cognitive impairment, low muscle mass and muscle weakness, osteoporosis, and abnormal weight may require a targeted approach to rehabilitation. Interestingly, anxiety, dyspnoea, and fear of physical activity have all been associated with underlying structural changes within the grey matter of the brain.23,24Encouragingly, however, studies have shown that patients with these psychological comorbidities respond better to pulmonary rehabilitation than those free from these conditions or with more physical complications. When tested, patients with the psychological cluster of symptoms demonstrated greater improvements in lung capacity and a higher likelihood of achieving meaningful improvements in the 6-minute walking distance than their counterparts without, or with more physical, comorbidities.25,26
Evidence confirms that pulmonary rehabilitation is as successful a strategy in AATD as it is in COPD. In a study of patients awaiting lung transplantation, pulmonary rehabilitation increased the 6-minute walking distance by 48 m in the AATD cohort, with 18% also experiencing an improvement in their mental quality of life measured by the SF-36 questionnaire.27Medication is another important player in pulmonary rehabilitation. For example, aclidinium bromide-induced bronchodilation significantly improved symptom-mediated activity limitation in a Phase III trial in COPD.28 Data from the large COPD Biomarker Qualification Consortium database of 1,200 patients investigated the average improvement in constant work rate cycle ergometer endurance in patients with COPD with different degrees of lung function impairment (Global Initiative for Chronic Obstructive Lung Disease [GOLD] category 1–4). While comparable increases in exercise tolerance in response to rehabilitative exercise training were achieved across the full range of lung function impairment, bronchodilator therapy became an important means to achieve a higher training capacity the higher the GOLD class.29However, average increases in exercise tolerance in response to rehabilitative exercise training showed equivalence across the full range of lung function impairment.29
A recent comparative study specifically evaluated the impact of training in nine patients with AATD versus ten with ‘usual’ COPD.30 Participants performed an incremental cycling test and underwent musculus vastus lateralis biopsies before and after a 3-week pulmonary rehabilitation programme including exercise training. Physical training and pulmonary rehabilitation were shown to improve physical performance in patients with COPD both with and without AATD; however, the maximum exercise capacity on the bike was found to be lower in patients with AATD, and adjustments in skeletal muscle were also different between the two groups. Overall, these findings suggest that disease-specific training may be required to optimise pulmonary rehabilitation in AATD. To address this question, a trial is currently ongoing evaluating a high-intensity approach in AATD that combines heavier weight loads with increased endurance goals.








