Meeting Summary
This symposium took place during the 2018 European Respiratory Society (ERS) International Congress in Paris, France and focussed on the disease burden associated with uncontrolled persistent asthma, particularly that driven by Type 2 inflammation; the impact of Type 2 cytokines on the pathophysiology of asthma and other Type 2 inflammatory diseases; current approaches to the assessment and management of uncontrolled persistent asthma; and future aspirations for treatment. Dr Kraft discussed the epidemiology, disease burden, and unmet medical needs of patients with uncontrolled persistent asthma. These patients have an increased risk of exacerbations, morbidity, mortality, and disease progression. Many patients have evidence of Type 2 inflammation, which constitutes a heavy disease burden and is further impacted by Type 2 inflammatory comorbidities. Prof Busse considered how Type 2 inflammation drives the key pathophysiologic characteristics of asthma. Persistent Type 2 inflammation and airway remodelling contribute to a self-perpetuating vicious cycle of exacerbations and progressive loss of lung function, and, therefore, impact disease progression. Targeting specific Type 2 inflammatory pathway cytokines reduces the pathophysiological impact of asthma and other Type 2 inflammatory comorbidities. Prof Pavord examined the current thinking around the management of uncontrolled persistent asthma driven by Type 2 inflammation. Despite recent advances in patient management, unmet needs remain. Of note, biologics have limitations and some patients are ineligible for currently vailable treatments.
Setting the Stage: The Current State of Uncontrolled Persistent Asthma with Type 2 Inflammation
Doctor Monika Kraft
Asthma is a prevalent and progressive disease characterised by recurring symptoms, variable airway obstruction, airway hyper-responsiveness, and chronic airway inflammation;1 it constitutes a heavy global burden and public health concern. In 2015, asthma was the most prevalent chronic respiratory disease, affecting an estimated 358 million people and accounting for 397,000 deaths worldwide.2 Its prevalence is also steadily rising, with an estimated global increase of 12.6% from 1990–2015.2
Asthma remains uncontrolled in many patients despite the use of current treatments,3 with recent European surveys estimating that 45–54% of patients have poorly controlled or uncontrolled asthma.3 Compared with patients with well-controlled asthma, patients with uncontrolled persistent asthma have an increased risk of exacerbations and hospitalisations; increased morbidity, mortality, and disease progression (as indicated by overuse of short-acting beta agonists);3-6 reduced and declining lung function;7 and impaired health-related quality of life.6 Moreover, uncontrolled persistent asthma is associated with the progressive development of Type 2 inflammatory comorbidities8 and the high use of oral corticosteroids (OCS).3 In the TENOR I study9 of 725 adolescents and adults with asthma, there was a 3.2-fold increased risk of hospitalisation, emergency department visit, or OCS burst in patients with uncontrolled disease versus those with controlled asthma. The same study also showed a significantly decreased post-bronchodilator percent predicted forced expiratory volume in 1 second (FEV1) for patients with uncontrolled disease versus those without (84.9 versus 67.6; p<0.0001).9 In the cross-sectional European National Health and Wellness Survey, having poorly controlled asthma (n=1,677) was associated with significantly worse mean 12-Item Short Form Health Survey component scores compared with patients with well-controlled asthma (n=1,480) for the physical (39.9 versus 48.0, respectively; p<0.001) and mental (40.6 versus 45.0, respectively; p<0.001) components.6 Similarly, in a UK survey, patients (n=246) with poorly controlled asthma reported significantly higher (worse) mean Marks Asthma Quality of Life Questionnaire scores than patients (n=412) with well-controlled asthma (17.5 versus 4.1, respectively; p<0.001).10
The Global Initiative for Asthma (GINA) 2018 guidelines11 recommend a stepwise approach to asthma treatment. The recommendations have been updated to include low-dose inhaled corticosteroids as an initial step, along with leukotriene receptor antagonists and tiotropium and/or biologics (anti-IgE, anti-IL-5) as subsequent treatment options. In patients with severely uncontrolled asthma or with acute asthma exacerbations, a short course of low-dose OCS is recommended as an add-on to regular treatment. However, it is not clear whether current therapies impact the risk of disease progression.
Long-term use of OCS is associated with various adverse effects, including muscle weakness, back pain, osteoporosis, cataracts, glaucoma, diabetes, hypertension, gastrointestinal bleeding, increased susceptibility to infection, and bruising.12-14 Side effects have also been reported with intermittent OCS use15 and managing these side effects is associated with a heavy economic burden. Data from the Severe Asthma Registry in Italy showed that among the 62% of patients with severe asthma who were receiving OCS, annual OCS-related events cost 193% more compared to the general population.16
More than 50% of patients with asthma have evidence of Type 2 inflammation in the airway,17,18 characterised by an early onset, the presence of atopic or allergic manifestations and eosinophilia, and responsiveness to corticosteroids.19,20 Markers of Type 2 inflammation include elevated fractional exhaled nitric oxide (FeNO), serum IgE, eosinophils, and chemotaxins (e.g., periostin, thymus and activation-regulated chemokine, and eotaxin-3).8,21-23 Type 2 inflammation is associated with a high disease burden. A study in 406 paediatric and adult patients with asthma found that elevated levels of local (FeNO) and systemic (blood eosinophils) Type 2 inflammatory markers correlated with a higher likelihood of moderate-to-severe bronchial hyper-responsiveness (BHR), assessed using the provocation dose of a bronchoconstrictor agonist, causing a 20% fall in FEV1 (PD20) <0.3 mg. The risk of BHR was greatest when both of these markers were elevated, compared with increased levels of either marker alone.24 Another prospective study in 304 patients with persistent asthma showed that FeNO levels >300% correlated with increased frequency of exacerbations requiring OCS use.25
Patients with asthma driven by Type 2 inflammation are at risk of comorbid Type 2 inflammatory diseases, including allergic rhinitis, atopic dermatitis, chronic rhinosinusitis (CRS) with or without nasal polyps, food allergy, eosinophilic oesophagitis, and aspirin or nonsteroidal anti-inflammatory drug-exacerbated respiratory disease.26-31 Type 2 inflammation underpins the pathophysiology of Type 2 inflammatory diseases (Figure 1) and Type 2 pathophysiologic features drive the clinical manifestations of Type 2 inflammatory disease, increasing the disease burden in patients with Type 2 comorbidities. Therefore, it is important that clinicians assess for such comorbidities and address their management, where possible, to maximise the chances of asthma control.
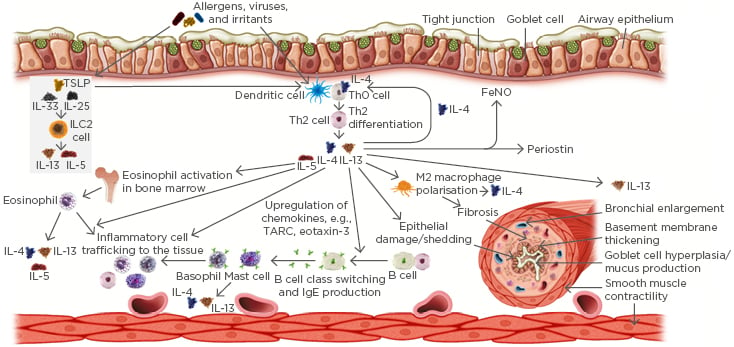
Figure 1: Common effects of Type 2 inflammation in the pathophysiology of Type 2 inflammatory respiratory diseases.
FeNO: fractional exhaled nitric oxide; ILC2: Type 2 innate lymphoid cells; TARC: thymus and activation-regulated chemokine; TSLP: thymic stromal lymphopoietin.
The presence of comorbid Type 2 inflammatory diseases is linked to an increased risk of asthma exacerbations. Data from 118,981 patients with asthma included in the Optimum Patient Care Research Database showed that rhinitis, CRS with nasal polyps, atopic dermatitis, and nasal polyps were all significantly associated with an increased likelihood of asthma exacerbations.32 Similarly, a study of 709 patients with asthma in the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 found that the annual rate of exacerbations in patients with severe asthma was significantly greater in those with comorbid sinusitis versus those without (4.4 versus 2.5, respectively; p<0.001).33 Another study of 79 adult patients with asthma showed that asthma control and lung function were decreased in patients with comorbid CRS with nasal polyps, compared to those without nasal polyps.34 Since uncontrolled persistent asthma presents challenges for disease control and management, treatments should address the full burden of the disease, from signs and symptoms to comorbidities.
Addressing the Key Issues in the Treatment of Uncontrolled Persistent Asthma with Type 2 Inflammation
Professor William Busse
The pathophysiology of asthma is underpinned by a complex interplay between inflammatory pathways involving multiple cytokines and inflammatory cells.35 As highlighted by Dr Kraft, >50% of patients with asthma have evidence of Type 2 inflammation in the airway.17,18 This is characterised by the presence of IL-4, IL-5, and IL-13, which are produced by Th2 cells and innate lymphoid cells in response to allergens, infectious agents, irritants, and pollutants.35 The components of Type 2 inflammation include airway inflammation, goblet cell hyperplasia, mucus production, smooth muscle contractility and proliferation, and airway remodelling. These components drive the key clinical characteristics of asthma, namely airway hyper-responsiveness, symptoms (e.g., reduced lung function), airway obstruction, and exacerbations.11,35
IL-4 and IL-13 are key Type 2 cytokines that drive many of the pathophysiologic features of asthma. For example, IL-13 contributes to goblet cell hyperplasia and increases expression of MUC5AC, which regulates epithelial cell mucus production,36,37 contributing to airflow obstruction.38 IL-13 also directly influences airway smooth muscle characteristics, causing increased contractility, decreased relaxation capacity, and potentiating mitogenic effects in the muscle,39-41 and influences airway fibrosis directly and indirectly via TGF-β.42,43 IL-4 and IL-13 contribute to epithelial barrier disruption by increasing epithelial permeability44,45 and are central to airway remodelling.19,46 Persistent Type 2 inflammation and airway remodelling contribute to a self-perpetuating vicious cycle of exacerbations and progressive loss of lung function, and, therefore, impact disease progression.19,47
Asthma is a heterogeneous disease with multiple phenotypes.19,20,48 In Type 2 asthma, high and low endotypes are recognised, reflecting a spectrum of inflammation.49 Type 2 cytokines regulate the production of inflammatory biomarkers in asthma. For example, IL-4 and IL-13 induce B cell class switching, which leads to an increase in IgE production and allergic sensitisation of mast cells and basophils that, when activated by specific allergens, cause allergic reactions.8 IL-5 promotes maturation and activation of eosinophils, and IL-4 and IL-13 contribute to eosinophil trafficking to the lung tissues via adhesion molecules (e.g., vascular cell adhesion molecule-1) and chemokines (e.g., eotaxin-3).8,50,51 IL-4 and IL-13 also induce FeNO production by airway epithelial cells.52
FeNO is a product of airway epithelium that is generated by inflammation and is increasingly recognised as a valuable biomarker of asthma driven by Type 2 inflammation. FeNO is a noninvasive objective measure that is quick and easy to perform. FeNO levels are elevated in asthma characterised by Type 2 inflammation and can remain high despite high- dose inhaled corticosteroid treatment in patients with uncontrolled or unresponsive asthma.11,52 FeNO, IgE, and eosinophil biomarkers are associated with overlapping and distinct asthma disease phenotypes (Figure 2A). Evidence shows that elevation of both FeNO and eosinophils has an additive effect on airway BHR (Figure 2B) and leads to higher exacerbation rates than elevation of either biomarker alone,24 highlighting the value of using a panel of biomarkers to characterise asthma with Type 2 inflammation.
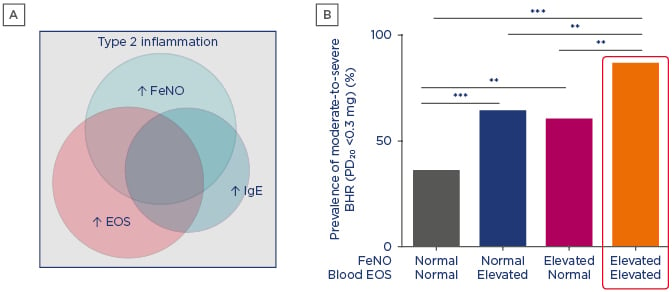
Figure 2: A) Asthma biomarkers are associated with overlapping and distinct disease phenotypes. B) Elevated fractional exhaled nitric oxide and eosinophil Type 2 biomarkers correlate with increased airway bronchial hyper-responsiveness.
**p<0.01; ***p<0.001.
BHR: bronchial hyper-responsiveness; EOS: eosinophils; FeNO: fractional exhaled nitric oxide.
A) Adapted from Spahn et al.;52 B) Adapted from Malinovschi et al.24
As discussed, Type 2 inflammation also underlies the pathophysiology of a number of inflammatory diseases that can be comorbid with uncontrolled persistent asthma.8,32 Many of the pathophysiologic features of asthma are also observed in these diseases, and a number of biomarkers are shared.8,53 For example, in CRS with nasal polyps, the effects of Type 2 inflammation include infiltration of immune cells, mucus production, microbiome alterations, barrier disruption, and tissue remodelling, which are associated with clinical disease manifestations, such as nasal polyps, obstruction, and discharge or post-nasal drip, which can lead to facial pain and a loss of smell.8,54-56 In allergic rhinitis, Type 2 inflammation is associated with infiltration of immune cells, IgE production and cross-linking, mucus production, and barrier disruption, leading to clinical consequences that include itchy eyes and nose, sneezing, nasal blockage, nasal discharge and post-nasal drip, and rhinorrhoea.8,57 A consequence of this common pathophysiology is that multiple comorbid Type 2 inflammatory diseases can develop over time.32,58 Hence, specifically targeting Type 2 inflammatory pathway cytokines reduces the pathophysiological impact of asthma and other Type 2 inflammatory comorbidities.
Management of Uncontrolled Persistent Asthma: Current Considerations and Future Possibilities
Professor Ian Pavord
For every patient, the goals of asthma management are to achieve symptom control, maintain normal activity levels, and minimise future risk of exacerbations, fixed airflow limitation, and treatment side effects. As discussed by Dr Kraft, GINA advises a stepwise approach to the use of treatments to achieve this.11
Patients with uncontrolled persistent asthma driven by Type 2 inflammation have a heavy disease burden associated with reduced lung function, increased exacerbations and hospitalisations, Type 2 inflammatory comorbidities, OCS use, and reduced health-related quality of life.3,7,9,13,34 Exacerbations have a direct, substantial impact on the economic burden of asthma. Results from a retrospective cohort study of 222,817 patients with asthma showed that the mean total asthma-related healthcare cost per exacerbation increased with disease severity and number of prior exacerbations in the 30 days post-exacerbation.59
The basic principles underpinning the management of uncontrolled persistent asthma are correct diagnosis, correct choice and use of treatment, and therapeutic adherence. Other considerations include the patient’s risk of exacerbations and the risk of progressive disease and future disability. Careful assessment of patients with uncontrolled asthma is necessary to identify the disease phenotype, taking into consideration objective evidence of asthma (i.e., airway hyper-responsiveness, reversible airflow obstruction, peak expiratory flow variability, and variable wheeze); evidence of airway inflammation (i.e., sputum eosinophilia or neutrophilia, blood eosinophilia, and increased FeNO); and any other factors contributing to their symptoms (i.e., predominant dry cough, fixed airflow obstruction, bronchiectasis, and extrapulmonary factors).11 Patients with uncontrolled persistent asthma driven by Type 2 inflammation may also have an increased prevalence of overlapping Type 2 inflammatory diseases, which further increases the disease burden and provides additional challenges for disease management.8,32
Multiple biomarkers can aid treatment decisions in patients with asthma driven by Type 2 inflammation, including FeNO and blood or sputum eosinophils,8,11 providing valuable information in addition to clinical assessment. A cross-sectional study of 12,408 patients demonstrated that elevated FeNO and blood eosinophil levels were associated with more asthma attacks and asthma-related emergency department visits compared with normal values,60 while the inclusion of three biomarkers (FeNO, blood eosinophils, and periostin) in a composite scoring system further differentiated patients on the basis of exacerbations, independent of asthma symptoms.61 These findings suggest that assessing a panel of Type 2 biomarkers, instead of isolated biomarkers, may better support asthma management.
Asthma phenotyping requires consideration of multiple factors, including clinical history and pattern of symptoms, comorbidities, and biomarkers.48 Patients with severe asthma predominantly have symptoms/airway hyper-responsiveness, Type 2 inflammation/exacerbations, or a mixture of the two.8,48 Both of these groups require a different approach to management. For example, for patients in whom symptoms/airway dysfunction are dominant (e.g., early symptom predominant or obese female non-eosinophilic asthma), therapy directed at airway hyper-responsiveness (e.g., tiotropium, bronchial thermoplasty), antineutrophil treatment (e.g., macrolide antibiotics), and addressing comorbidities are considered effective approaches. In comparison, for patients in whom inflammation/risk of exacerbation are dominant (i.e., inflammation-predominant asthma), targeted biologic Type 2 therapy should be considered.8,48
Four biologic therapies active against Type 2 inflammation are currently licensed in the European Union (EU) and the USA for the treatment of asthma. Omalizumab is a humanised anti-IgE monoclonal antibody (mAb) that specifically binds free IgE in the serum and can interrupt the allergic cascade by preventing binding of IgE with its high-affinity FcεRI receptors on mast cells, antigen-presenting cells, basophils, and other inflammatory cells.62 The other three licensed biologics target IL-5 signalling, binding to either IL-5 directly (the humanised mAb mepolizumab and reslizumab) or to the IL-5 receptor (the fully human mAb benralizumab), resulting in a depletion of blood and airway eosinophils and basophils.63 Agents undergoing Phase III clinical testing for poorly controlled/uncontrolled, persistent, or severe asthma include tezepelumab (a human mAb that specifically targets the epithelial cell-derived cytokine, thymic stromal lymphopoietin) and dupilumab (a fully human mAb that specifically binds to the alpha subunit of the IL-4 receptor-alpha). Although targeted therapy based on patient characteristics has led to improved outcomes in patients with uncontrolled persistent asthma driven by Type 2 inflammation, unmet needs remain.
Current biologic therapies are indicated for asthma with an eosinophilic (IL-5) or allergic (IgE) phenotype only, leaving some patients ineligible for treatment and, thus, suboptimally controlled. In addition, these therapies only partially inhibit Type 2 inflammation and may be less effective than biologics that have a broader effect on signalling pathways and iomarkers of Type 2 inflammation. They have also not demonstrated consistent, clinically meaningful improvements in key asthma control goals across a broad range of Type 2 biomarkers, and no therapy addresses all common Type 2 comorbidities.8
Tools are now available that offer the potential to prevent asthma exacerbations64 and modify the course of the disease (Figure 3). Once a diagnosis of asthma is established, patient classification reflects disease heterogeneity and the biological basis of this heterogeneity using clinical and/or molecular phenotypes. Risk factors for abnormal lung growth and poor lung function include low FEV1, airway hyper-responsiveness, and increased OCS use, which can be modified with treatment.65 Frequent exacerbations are associated with a progressive loss of lung function and, therefore, reducing exacerbations may prevent or reduce this process.66 Interventions for established disease could be targeted at patient subpopulations most at risk of exacerbations, for example, patients with uncontrolled persistent asthma driven by Type 2 inflammation.
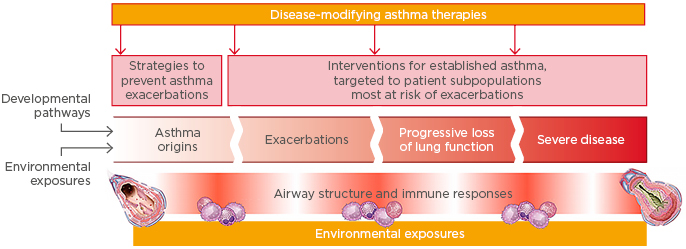
Figure 3: Is there potential to intervene to prevent asthma exacerbations and modify disease progression?
Adapted from Levy et al.64
The era of biologics has raised treatment expectations in asthma and changed future aspirations for treatment. Targeting the underlying pathophysiology of the disease may arrest or reverse progressive loss of lung function and airway structural changes, affording the potential for disease modification in uncontrolled persistent asthma.64
Conclusion
Asthma is a prevalent and progressive disease that remains uncontrolled in many patients, despite the use of current treatments. Many patients with uncontrolled persistent asthma have evidence of Type 2 inflammation. Persistent Type 2 inflammation and airway remodelling contribute to a self-perpetuating vicious cycle of exacerbations and progressive loss of lung function, and, therefore, impact disease progression. These patients are also at risk of comorbid Type 2 inflammatory diseases, which further contribute to the heavy burden of disease. Type 2 inflammation is complex and heterogeneous and encompasses multiple asthma phenotypes. Asthma phenotyping requires consideration of multiple factors, including clinical history and symptom pattern, comorbidities, and multiple biomarkers, such as FeNO, IgE, and eosinophils.
Targeting specific Type 2 inflammatory pathway cytokines reduces the pathophysiology of asthma and other Type 2 inflammatory comorbidities. However, despite recent advances in the management of uncontrolled persistent asthma driven by Type 2 inflammation, unmet needs remain. Of particular note, existing biologics have some limitations and not all patients are eligible for currently available treatments.
SAGLB.AST.18.10.1324
Intended approval date: October 2018







