In December 2017, the National Institute for Health and Care Excellence (NICE) updated its interventional procedures guidance, titled ‘Endobronchial valve insertion to reduce lung volume in emphysema’,1 to support the routine use of endobronchial valve therapy for emphysema. The NICE guidance states: ‘Current evidence on the safety and efficacy of endobronchial valve insertion to reduce lung volume in emphysema is adequate in quantity and quality to support the use of this procedure provided that standard arrangements are in place for clinical governance, consent and audit.’ NICE is the latest organisation to include endobronchial valves in its chronic obstructive pulmonary disease (COPD) treatment pathway, following the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report for the diagnosis, management, and prevention of COPD;2 the German Society for Pneumology and Respiratory Medicine (DGP) guidance;3 and the Australian and New Zealand guidelines for the management of COPD;4 however, what does this mean to us as respiratory physicians?
Let us start by looking at emphysema. The British Lung Foundation (BLF) estimates that 1.2 million people in the UK are living with diagnosed COPD and almost 30,000 patients die from the disease every year.5 Roughly one- third of COPD patients have the emphysema subtype of COPD, presenting with destruction of the lung parenchyma.6 These patients are chronically breathless, making even the most simple daily activities, such as washing, eating, walking, and household chores, difficult and often leading to patients becoming housebound and depressed. In fact, the quality of life of emphysema patients is commonly worse than Stage IV lung cancer patients.7
Current emphysema treatments range from conservative medical management to invasive lung volume reduction surgery and, ultimately, lung transplantation. Strategies involving endobronchial valves, namely bronchoscopic lung volume reduction (BLVR), provide a less invasive, nonsurgical approach to treatment. During BLVR, tiny endobronchial valves are implanted via a bronchoscopic procedure in the airways of the more diseased areas of the lung (target lobe) to block air from entering. This allows the target lobe to collapse, reducing overall hyperinflation, improving breathing mechanics, and enabling healthier areas of the lungs to expand and take in more air.
We have been using endobronchial valves to treat emphysema for almost 18 years and have been closely involved in developing the extensive body of clinical evidence available today for the leading endobronchial valve, the Zephyr® Endobronchial Valve (Pulmonx Corp., Redwood City, Califonia, USA). The Zephyr Endobronchial Valve is available in four sizes: 4.0, 4.0 LP (for an airway diameter of 4.0–7.0 mm), 5.5, and 5.5 LP (for an airway diameter of 5.5–8.5 mm).
Ideally, the treatment target during BLVR is the most diseased lobe of the lung. However, the VENT study,8 which was the first randomised clinical trial of the Zephyr Valve, provided important insights regarding appropriate patient selection for this therapy, specifically the need to treat patients with little-to-no collateral ventilation between the target lobe and the adjacent lobe and to achieve total lobar occlusion.8 Collateral ventilation in the human lung refers to the ventilation of alveolar structures through passages or channels that bypass the normal airways.9 In the presence of collateral ventilation, the occluded lobe fails to achieve atelectasis because of continuous air entering the lobe through the collateral channels. The availability of diagnostic tools, such as the Chartis® Lung Assessment System (Pulmonx Corp.), which provides a physiological measure of airflow from a lobe occluded with a balloon10 and/or quantitative CT assessment of fissure integrity (a surrogate for collateral ventilation),11 enables the selection of patients who are most likely to benefit from this therapy. In our experience, the use of these tools generally achieves a 70% response rate in improving lung function and, more importantly, a clinically meaningful response, with patients being able to take part in the activities that matter to them with less breathlessness.
The NICE committee further noted that “there are different devices available for this procedure, and that the published evidence shows they may have different efficacy profiles.”1 NICE assessed four randomised clinical trials12-15 of the Zephyr Valve, which showed consistent improvement in breathing function, exercise capacity, and quality of life, and one randomised controlled trial conducted for another valve, the IBV Valve (Spiration Inc., Redmond, Washington, USA),16 which showed statistically significant improvements in the coprimary endpoints (the number of patients with both a reduction in target lung volume and improvement in forced expiratory volume in 1 second). However, for the participants of the latter study, changes in quality of life and lung function were modest, not clinically meaningful, and not significantly different from patients in the placebo group, possibly because the treatment was non-lobar.16
The efficacy data for the Zephyr Valve are comparable to that of lung volume reduction surgery (Figure 1) but with lower morbidity and mortality,17-19 making valves the first choice treatment option. The Zephyr Valve is also removable, thus allowing the procedure to be reversed if a patient does not respond or has complications. BLVR with a Zephyr Valve does not negate the option of surgical lung volume reduction, if desired. NICE recommends that patient selection for BLVR with valves should be performed by a multidisciplinary team experienced in managing emphysema, which should typically include a chest physician, radiologist, thoracic surgeon, and a respiratory nurse.
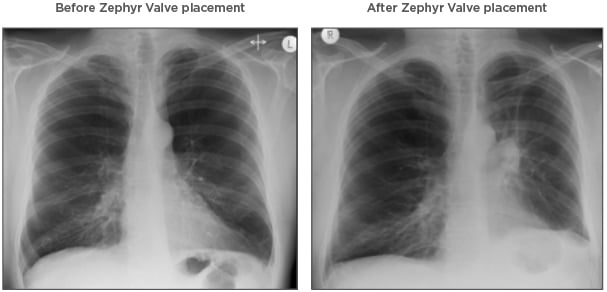
Figure 1: Chest X-ray images before and after Zephyr® Valve treatment.
The chest X-ray images highlight the reduction in hyperinflation and return of the diaphragm to a near-normal position with Zephyr Valve placement.
Images courtesy of Hugo Goulart de Oliveira, Porto Alegre, Brazil.
The benefits provided by any treatment must be balanced against the safety profile associated with the procedure. The most frequent side effects associated with BLVR with a Zephyr Valve are pneumothorax (up to 26%) and COPD exacerbations (up to 77%) in the periprocedure period.1,11,12 Targeted lobar deflation likely causes inflation of the ipsilateral lobe, which can lead to a tear of the already compromised parenchymal tissue of the emphysematous ipsilateral lobe, resulting in a pneumothorax, which is managed with standard techniques. Most pneumothoraces occur within the first 2–3 days postprocedure; as a result, a minimum of a 3-night hospital stay is recommended for patients undergoing BLVR with valves. In this severely affected emphysema patient group, the overall benefit–risk profile is in favour of valve therapy. NICE agrees with this assessment, stating that there are no serious concerns about its safety.1 Patients considering this treatment option should be informed of the potential risks and benefits of the procedure to make an informed decision.
While many physicians are satisfied with the available data on valves, NICE required a preponderance of consistent data before recommending valves as a treatment option. Their detailed assessment of all available data allowed NICE to reach the same conclusion as physicians: patients treated with endobronchial valves experience tangible benefits through improvements in their lung capacity, quality of life, and/or level of breathlessness.
This NICE decision is a huge step forward because health authorities will now recognise that BLVR with valves is an effective therapy and has the potential to meaningfully improve lung function, dyspnoea, exercise capacity, and quality of life for emphysema patients. General practitioners should consider referring patients with COPD who present with breathlessness while performing normal day-to-day activities, such as walking or doing light chores, despite medical management, for further evaluation and potential consideration for this treatment. While the NICE recommendation for the routine use of valves does not guarantee that health authorities will fund the therapy, it certainly facilitates the process and can be very meaningful to pulmonologists and patients. More recently, in June 2018, the U.S. Food and Drug Administration (FDA) also approved the Zephyr Valve for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little-to-no collateral ventilation,20 validating its safety and efficacy.
Previously, when I have applied for exceptional funding to treat patients with valves, I would apply for funding for 20 well-selected patients and receive approval for only a few. Now, with the NICE recommendation, it will likely be much easier; I now expect to get approval for perhaps 16 or 17 patients out of 20. The UK health authorities generally follow the lead of NICE and, therefore, this decision has large potential to increase patient access to this important technology. As a physician who has been involved with valves since the beginning, it is very gratifying to have seen the positive trajectory for this treatment over recent years. It is even more exciting to see the life-changing impact it can have for patients and their families. I applaud NICE on their decision and encourage private insurers to take the lead in making this technology available to improve the lives of very sick emphysema patients.








