Abstract
Human toxocariasis is a zoonosis caused by the larvae of Toxocara genus parasites. It is usually asymptomatic and self-limiting. However, due to either the direct action of parasites or by immunological mechanisms, it can affect several organs resulting in many clinical manifestations. Among paediatric patients, lung involvement occurs in 20–85% of cases of visceral toxocariasis, as Löffler’s syndrome, chronic pneumonia, eosinophilic pneumonia, or baby wheezing syndrome. Because of its rarity, eosinophilic pneumonia due to Toxocara larvae is not well-documented amongst medical records.
This article presents a clinical case of a 2-year-old with a history of daily sand and soil ingestion, followed by sudden pulmonary symptoms, 9-day fever, abnormal chest X-ray, and intense peripheral eosinophilia. Due to the suspicion of toxocariasis pneumonia after a series of laboratory tests, the diagnosis of eosinophilic pneumonia caused by the parasite was confirmed. After treatment with albendazole for 5 days, the patient displayed progressive improvement in respiratory symptoms and a reduction in peripheral eosinophilia.
Key Points
1. Human toxocariasis is a zoonotic infection that occurs secondary to ingestion of infective larvae following contact with contaminated soil. It is usually an asymptomatic and self–limiting illness.2. In children, lung involvement occurs in 20–85% of visceral toxocariasis cases. Eosinophilic pneumonia secondary to toxocariasis, most commonly caused by Toxocara canis or Toxocara cati, is a rare complication.
3. Diagnosis is made through a combination of clinical history, physical examination, serology, radiology, and bronchoalveolar lavage results. Treatment with anthelmintic medication leads to symptom improvement and reduction in eosinophilia.
INTRODUCTION
Human toxocariasis is a zoonosis caused by parasite larvae of Toxocara spp. The most prevalent pathological species are Toxocara canis and Toxocara cati, intestinal parasites from dogs and cats, respectively.1
Reported prevalence of this zoonosis varies geographically. It has a relatively high prevalence in tropical areas worldwide, mostly in regions with low socioeconomic levels, low urbanisation, and with poor access to sanitary conditions.1,2 Human beings, especially children, infect themselves after contact with contaminated soil found mainly in parks and gardens, and they subsequently ingest infective eggs by habitually putting their hands in their mouths.3
Toxocariasis is usually an asymptomatic and self-limiting disease; however, it can cause various manifestations according to the organs affected.1 Amongst children, lung involvement can occur in up to 20–85% of visceral toxocariasis cases, with presentations such as Löffler’s syndrome, chronic pneumonia, eosinophilic pneumonia, and wheezing.4,5
Eosinophilic pneumonia is a heterogeneous group of lung diseases characterised by infiltration of lung parenchyma by eosinophils, resulting in alveolar eosinophilia (>25%) and pulmonary infiltrates, with or without evidence of peripheral blood eosinophilia (>1×109 eosinophils/L). Its precise incidence is unknown, estimated at <0.1 cases/100,000 inhabitants per year.6
Parasitic infestation is the main cause of eosinophilic pneumonia worldwide. Clinical manifestations are nonspecific and can range from asymptomatic pulmonary infiltrates to respiratory failure, requiring application of appropriate methods to establish the diagnosis.4
The present study reports a clinical case of a paediatric patient with eosinophilic pneumonia secondary to toxocariasis and provides a literature review on this subject.
CASE DESCRIPTION
A 2-year-old male, with a previous diagnosis of epilepsy, developed a 9-day fever with associated cough and dyspnoea. Following emergency services evaluation, the patient was noted to have an increased work of breathing, diffuse wheezing, and crackles, and was subsequently hospitalised.
During hospital stay, the patient’s chest X-ray revealed diffuse interstitial infiltrate and laboratory findings showed significant peripheral eosinophilia (Table 1). Following these results, the hypothesis of T. canis infection was raised, especially after the mother’s narrative that the patient had a daily habit of eating sand from their back garden.
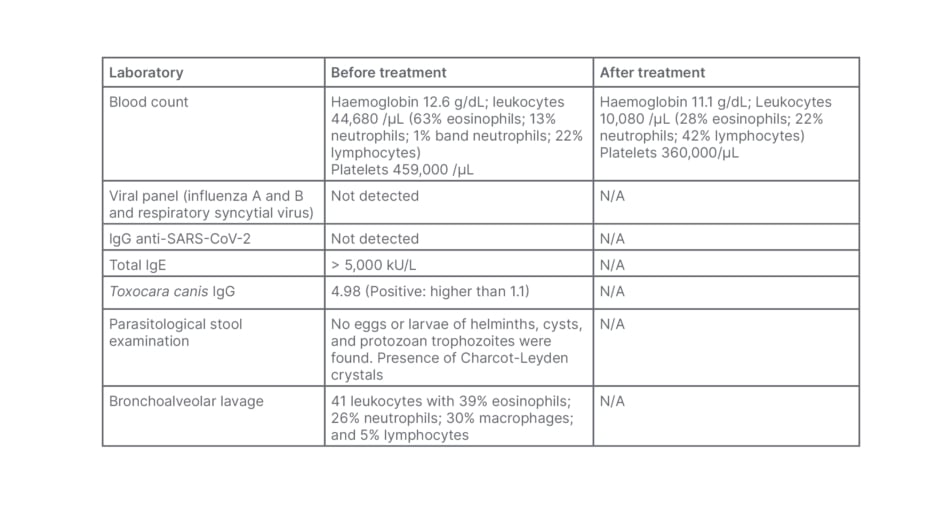
Table 1: Laboratory findings of the patient before and after treatment.
N/A: not applicable; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
The diagnosis was confirmed with positive serology (IgG) for toxocariasis (Table 1). The possibility of systemic involvement of this condition was investigated, and an echocardiogram and abdominal ultrasound were performed, without significant abnormalities. A chest CT scan (Figure 1) showed diffuse involvement of centrilobular nodules combined with areas of ground glass opacity. As a result of this pulmonary involvement, bronchoalveolar lavage (BAL) was performed (Table 1), which showed peripheral eosinophilia compatible with eosinophilic pneumonia secondary to toxocariasis. The patient was treated with albendazole for 5 days, resulting in a reduction in peripheral eosinophilia and progressive clinical improvement. The patient remains under outpatient follow-up with paediatric pulmonology, with ongoing reduction in eosinophilia, and no respiratory symptoms or sequelae of any kind.
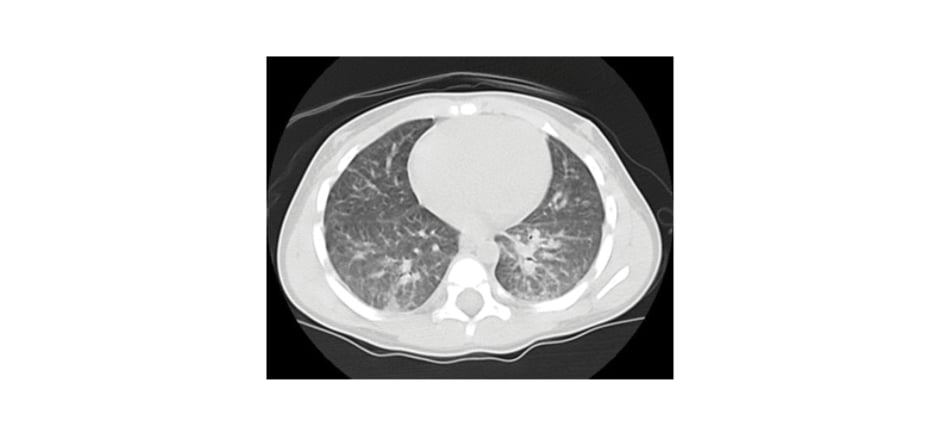
Figure 1: CT scan of the patient revealing ground glass opacity, nodules and micronodules, and bilateral peribronchovascular interstitial thickening.
DISCUSSION
Pathophysiology
The most prevalent species of Toxocara responsible for visceral symptomatic infections among humans are T. canis and T. cati. The definitive hosts are usually the domestic dog or cat and, when infected, they eliminate adult worms spontaneously. Female worms produce thousands of eggs that are resistant to hostile factors in the external environment. When favourable conditions of humidity and temperature occur, those eggs are embryonated and become infectious. Domestic pets become infected by ingestion of embryonated eggs, ingestion of the larvae, transplacental migration, and transmission by colostrum during lactation amongst other causes. Infections in children usually develops after the ingestion of eggs secondary to contact with infected pups or contact with objects containing eggs or larvae.2
After ingestion of Toxocara eggs, the larvae hatch and migrate to different tissues, resulting in a systemic inflammatory reaction.7,8 Symptoms vary according to the host’s immune response and the number and location of these larvae. In individuals infected by a large volume of Toxocara eggs, there is significant eosinophilia and retention of large amounts of the parasite in liver and lung tissue, causing classic manifestations of Toxocara pneumonia.9 Pulmonary involvement of Toxocara is uncommon and responsible for causing a local allergic reaction after parasite infiltration. Lung injuries are more frequent in children under 1 year of age.7
One of the pulmonary affections related to infection by T. canis is eosinophilic pneumonia, which occurs due to several factors, including Type I hypersensitivity reaction, activated by alveolar macrophages following an external trigger; direct action of the infectious agent; or resulting from an inflammation cascade triggered by inflammatory cytokines, which cause eosinophilic recruitment.9,10 Some parasites bind to alveolar or parenchymal epithelial cells, causing the release of IL-33 and thymic stromal lymphopoietin, which are potent triggers of the Th2 immune response, responsible for the recruitment of eosinophils and T-helper cells.10
The mechanisms of lung injury caused by eosinophils are not fully understood. Eosinophilic inflammation is responsible for the release of granules between alveolar spaces and lung parenchyma, causing direct damage to the tissue. Other cells such as neutrophils, macrophages, and lymphocytes can also activate similar inflammatory responses; however, their role is not well understood.10
Clinical Manifestations
The majority of Toxocara infections are asymptomatic and systemic toxocariasis occurs in approximately 15% of diagnosed cases.2 The current classification of toxocariasis divides it into asymptomatic, systemic, compartmentalised (ocular and neurological), and covert. Ocular and neurological manifestations are probably the final stages of larvae migration, a consequence of non-treated toxocariasis complications.2 The visceral larva migrans syndrome is the most severe systemic form of toxocariasis, with fever, hepatosplenomegaly, leukocytosis, and high eosinophilia. The possible complications of prolonged extreme eosinophilia are eosinophilic myocardial or pulmonary fibrosis.2
Pulmonary toxocariasis is usually asymptomatic or causes only mild, non-specific symptoms. The classic, or symptomatic, form of the disease manifests itself in children with a previous history of geophagia. It starts with fever, hyporexia, and cough, mainly at night. On physical examination, there are commonly changes in respiratory auscultation, especially in the middle and lower lung lobes, with wheezing and/or crackles, similar to the physical examination of the patient in this case report. The condition may be accompanied by cervical adenomegaly, moderate hepatomegaly, and/or mild splenomegaly. In rare cases, it may progress to acute respiratory distress syndrome.6,11
In a study of 119 children positive for Toxocara serology, 23 children had chest X-ray abnormalities. Among them, only 15 children presented with clinical respiratory complaints such as chronic cough, wheezing, asthma, or haemoptysis. Cough (67%) was the most common symptom among patients. Other symptoms included chronic wheezing (47%), dyspnoea (13%), haemoptysis (7%), and general weakness (7%).7
The severe pulmonary manifestations seen in this case can be considered exceptional.8 There may be complications from prolonged eosinophilia, more common in cases of the classic (or symptomatic) form, such as pulmonary fibrosis.2
Diagnosis
The diagnosis of Toxocara eosinophilic pneumonia is based on the characteristic findings in the BAL associated with a positive anti-Toxocara ELISA serological test, in addition to the clinical and radiological findings suggestive of the disease.12 Definitive diagnosis is made through microscopy evaluation of infected tissues with direct visualisation of Toxocara larvae. It is the gold standard diagnostic test, but also an invasive method. Moreover, sometimes the larvae may not be found in the eosinophilic granuloma tissue wedge.13,14 Currently, the most used method for the diagnosis of human toxocariasis is the serological test anti-Toxocara ELISA, which detects antigens excreted and secreted by Stage 3 larvae of T. canis (Toxocara excretory-secretory antigen-based ELISA). Studies have shown that this test has 78% sensitivity and 92% specificity.13,14
There are other laboratory findings that can help in diagnosis, such as hypergammaglobulinemia (increased isohemagglutinin A and B), anaemia, and leukocytosis with marked eosinophilia.14 However, there are cases in which eosinophilia may be minor or absent. This situation may occur in milder and chronic cases.15 Peripheral eosinophilia is established when the eosinophil count is above 500 cells/mm3 and intense eosinophilia when above 1000 cells/mm.3 In cases with pulmonary involvement, there may be eosinophilia in the BAL.8,16
Eosinophilic pneumonia encompasses a heterogeneous group of diseases that are characterised by the presence of one or two criteria: pulmonary infiltrate with blood eosinophilia or eosinophilia on lung biopsy or BAL.16 Pulmonary eosinophilia most often occurs due to migration of the larval stage to the lungs, and is defined by an eosinophil count of greater than 5% in BAL, with intense eosinophilia when the eosinophil count is greater than 25%.16 The diagnosis of eosinophilic pneumonia is made when the level of eosinophils in BAL exceeds 25%, as in the reported clinical case.17 Among other causes of eosinophilic pneumonia, it is important to investigate other types of infections, such as fungal or parasitic, ingestion of drugs or toxins, smoking or other inhalational substance exposure, and multisystemic diseases such as eosinophilic granulomatosis with polyangiitis. It is fundamental to differentiate eosinophilic pneumonia from other distinct types of pneumonia, because early treatment of the underlying condition has an impact on patient outcome.10
Lung biopsy may be necessary in more complex cases with differential diagnoses for instance, to rule out neoplasms or other infections.16 Studies suggest that performing the serological ELISA test for Toxocara in patients with pulmonary infiltrate associated with eosinophilia may demonstrate a higher-than-expected number of cases of Toxocara pneumonia.12
Treatment
Even though the majority of patients with toxocariasis have a favourable prognosis, the larvae can remain in the human body for approximately 2 years. All hosts can suffer from new infective reactivation and larvae migration into brain or ocular tissues. Treatment is recommended for all symptomatic patients and helps to prevent the aforementioned complications.2
Studies on the topic of pharmacology treatment consist of the administration of anthelmintic drugs, such as albendazole at a dose of 400 mg twice per day for 5 days.18 Mebendazole 100–200 mg twice per day for 5 days is an alternative, but abendazole is preferred as it crosses the blood–brain barrier.19 Ivermectin does not appear to be effective for toxocariasis.20 Diethylcarbamazine at a dose of 3–5 mg/kg per day for 21 days was effective in a small number of cases, but it has potential adverse effects, and therefore is not widely used. Prospective studies are needed to evaluate the best drug therapy and its duration.20
The efficacy of treatment is difficult to evaluate because of toxocariasis subtle symptomatology. It remains unclear if reduction in hepatomegaly and/or eosinophilia can indicate reduction of larvae infectivity. Some serology markers can be helpful to demonstrate successful treatment, combined with eosinophil count and serum IgE antibodies.2 After a series of experimental studies and drug trials among paediatric and adult populations, high rates of successful therapy for symptomatic toxocariasis are well established, mainly with anthelmintic drugs. In contaminated environments, it is more common to see multiple reinfections rather than untreatable infections.21
Prevention is the most important strategy and includes good hygiene practices, appropriate disposal of pet faeces, and regular deworming of pets. Hand washing should be encouraged after contact with pets or areas at high risk of soil contamination such as litter boxes.15,21
CONCLUSION
The presentation of this article aimed to report a paediatric case of eosinophilic pneumonia secondary to toxocariasis, an uncommon condition with few well-documented medical records. The report brings to light the importance that for a good clinical outcome in this condition, it is necessary to correlate clinical history, physical examination, laboratory tests (blood count, serology for Toxocara, BAL), and imaging (chest radiography and CT). With a well-established diagnosis and adequate treatment, the patient’s clinical improvement is remarkable, making it possible for the child to return to their usual daily activities with no pulmonary symptoms or impairment.








