Abstract
Mucormycosis is a life-threatening fungal infection usually seen in patients who are immunocompromised; however, to date, it has been rarely described in patients with COVID-19, although more recently, reports from India have described an increased incidence of these infections. This report describes a patient with COVID-19 who developed a fatal dermatologic mucormycosis infection. The patient, whose history included therapy for diffuse large B-cell lymphoma, had an incidental positive screening test for COVID-19 in February 2021 after exposure to a family member who had tested positive. They then presented to the emergency room a few weeks later exhibiting progressive dyspnoea and fever. A CT scan of the chest revealed ground glass opacities. They were intubated approximately 2 weeks later and their course was complicated by renal failure, for which continuous renal replacement therapy was started, and by refractory hypoxaemic and hypercapnic respiratory failure in which they were placed on venovenous extracorporeal membrane oxygenation. During their course in the intensive care unit (ICU), they developed a right thigh haematoma, thought to be related to the previous insertion of a femoral arterial pressure monitoring catheter. Several days before the patient’s death, the wound noted to be covered by a brown-black eschar was cultured on April 30 and returned positive for Rhizopus oryzae and Staphylococcus epidermidis on May 5. The patient was immediately started on liposomal amphotericin and posaconazole and taken urgently taken to the operating room for a radical debridement. Unfortunately, their post-operative course was characterised by fulminant haemodynamic collapse and multiple system organ failure, from which the patient died.
Key Points
1. The authors examine the case of a rare co-infection between mucormycosis, a life-threatening fungal infection, and COVID-19 in a 54-year-old male.2. Invasive fungal infection such as mucormycosis often presents in one of four clinical syndromes, which affect the lungs, skin, sinuses, or presents as disseminated disease.
3. Cutaneous mucormycosis in patients with COVID-19 has a significant mortality rate, requiring swift diagnosis and immediate treatment.
CASE REPORT
A 54-year-old male tested positive in late February on a screening COVID-19 test performed after a family member’s test had become positive. A few weeks later, they presented to the emergency room at a different hospital centre with shortness of breath and fever. Their oxygen saturation in room air was 63%. A CT scan of the chest confirmed bilateral ground glass opacities with intralobular and interlobular septal thickening, predominantly in the lung bases. They were transferred to the intensive care unit (ICU), where they were intubated several days later for worsening respiratory failure.
The patient had been diagnosed with follicular lymphoma in 2015, which later that year had transformed into diffuse large B-cell lymphoma. The patient subsequently underwent several courses of chemotherapy in 2018–19, including a rituximab-cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone (R-CHOP) regimen; rituximab-gemcitabine-dexamethasone-cisplatin (R-GDP) chemotherapy; polatuzumab vedotin; and finally chimeric antigen receptor T-cell (CAR-T) therapy, the latter in August 2020. A PET scan in 2020, however, demonstrated an excellent response, albeit with some residual foci in the left axilla and right thigh. Three months later, a follow-up PET scan showed a slight progression of the disease in the right thigh.The patient also had laryngeal cancer, which had been treated with radiotherapy in 2012.
Therapy in the ICU at the referring center included empiric therapy with piperacillin plus tazobactam, azithromycin, remdesivir, and dexamethason. Additionally, trimethoprim plus sulfamethoxazole, later switched to atovaquone and primaquine plus clindamycin, had been introduced given concerns over the possibility of Pneumocystis jiroveci pneumonia. Intravenous immunoglobulin therapy was also administered given concerns over B-cell dysfunction consequent to the CAR-T therapy.
The patient was transferred to the authors’ centre for treatment of their worsening respiratory failure, and was subsequently placed on venovenous extracorporeal membrane oxygenation in early April. Cannulas were inserted in the right internal jugular and left femoral veins. An arterial pressure monitoring line was inserted into the right femoral artery from which the patient bled, developing a visible superficial haematoma on the right thigh, approximately 20 cm by 12 cm. A CT scan of the right thigh on April 28 revealed ill-defined fat stranding of the subcutaneous tissues without any evidence of either discreet collections or gas. The patient’s condition stabilised but the wound, covered by a brown-black eschar, failed to heal. A culture of the wound, taken on April 30, was reported on May 5 to be growing Rhizopus oryzae and Staphylococcus epidermidis.
The patient was immediately started on liposomal amphotericin and then taken to the operating room that evening with a diagnosis of tissue necrosis secondary to mucormycosis (Figure 1). A diagnosis suspected on visual inspection; however, when a radical debridement of the right thigh was performed diagnosis was confirmed. Unfortunately, that procedure was followed soon thereafter by distributive haemodynamic collapse requiring very large doses of vasopressors and inotropes. Posaconazole was added, but the patient ultimately died from multiple system organ failure.
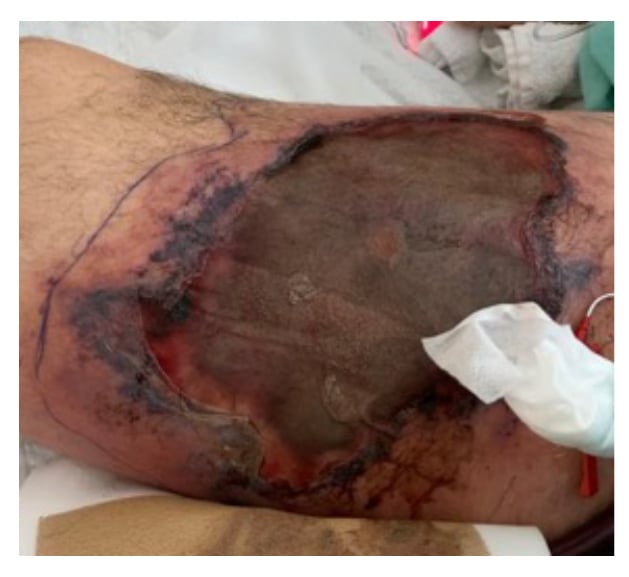
Figure 1: Cutaneous mucormycosis infection, 20 cm by 12 cm wound on right thigh of a COVID-19 positive 54-year-old male with cutaneous mucormycosis co-infection 3 months following ICU admission.
DISCUSSION
While opportunistic fungal infections, primarily Aspergillus superinfection of the lungs,1 have emerged as a growing clinical problem in patients with COVID-19, mucormycosis remains uncommon in this patient population, albeit a growing concern.2,3
Mucormycosis refers to the invasive fungal infection by saprophytic environmental fungi most usually, as in this case, by Rhizopus although other related fungi such as Mucor and Cunninghamella have also been identified. Such invasive fungal infections usually present in one of four clinical syndromes involving the sinuses, the lungs, the skin, and as disseminated disease, although gastrointestinal tract involvement has also been reported.4,5 Usually cited risk factors for these infections include uncontrolled diabetes, haematologic malignancies, and immune-incompetence. This includes patients with both solid and haematologic transplants, those receiving immunocompromising medications such as systemic steroids, and those patients exposed to potential nosocomial sources, in particular bandages and intravascular devices. Certain studies have linked specific risk factors with a particular clinical presentation.5 The four cornerstones of therapy include: rapid diagnosis which presupposes a high index of suspicion; reversal of underlying predisposing factors, if possible; appropriate antifungal medication; and surgical debridement, the latter deemed necessary given the propensity of this organism for angio-invasion, resulting in necrosis and poor drug penetration into devitalised tissues.2,4 Mortality is high, ranging from 30–50%, with increased survival associated with a longer duration of antifungal therapy and combined medical-surgical treatment.2,4,5
Mucormycosis, specifically in patients with COVID-19 infections, is still relatively uncommon, but is subject to an increasing number of clinical reports.2,3 Most recently, in addition to European countries,6 it has been reported that patients with COVID-19 in India are presenting primarily with rhino-orbital or rhino-orbital-cerebral infections, but reports of life-threatening infections of the skin by this fungus in the patient population remain rare. In their report, Khatri et al.7 described a heart transplant patient who, after developing COVID-19, died after contracting cutaneous mucormycosis. In their review of the literature, they identified an additional seven cases of mucormycotic superinfection in patients with COVID-19, none of whom had cutaneous mucormycosis, but all of whom died.7
This patient did not suffer from diabetes. In fact, their blood glucose was relatively well controlled between 5.5–7.8 mmol/L for most of their stay in the ICU. However, it must be recalled that the haematoma had been related to the insertion of an arterial catheter, an intravascular device, identified as a risk factor for this infection.
However, the patient likely did suffer from a significant level of immune incompetence. Firstly, COVID-19 infection itself has been linked to immune suppression.8 Although they never received tocilizumab, they did receive steroids as a part of their anti-COVID-19 treatment. Parenthetically, in this regard, PCR of the patient’s lower respiratory tract secretions continued to be positive for COVID-19 throughout the entire 70-day course of illness. The authors are unable to comment on the status of the patient’s underlying malignancy; however, it is a possible contribution to their impaired immune status based on the PET scan findings in 2020 that showed increased right thigh activity. Although the patient had received significant immunosuppressive chemotherapy during 2019, the authors believe it unlikely that the immunosuppression resulting from those treatments played a significant role so long after their administration.
However, this patient had received CAR-T therapy in August 2020, approximately 7 months prior to the onset of this opportunistic fungal infection. CAR-T therapy is a relatively new immune effector cell therapy used primarily in refractory or relapsed haematologic malignancies such as in diffuse large B-cell lymphoma, similar to this patient’s case. B-cell aplasia is usually a delayed complication of this therapy and is reported to persist for as long as 3 years following therapy.9 Given the relative newness of this therapy, the risks of delayed infections have yet to be clearly elucidated; however, recent studies have pointed to several risk factors including the incidence of cytokine release syndrome immediately accompanying CAR-T therapy, the presence or absence of haematopoietic transplantation, the type of accompanying chemotherapeutic agents, and the persistence of hypogammaglobulinaemia. As noted above, this patient had received intravenous immunoglobulins during their most recent hospitalisation. One study cited the cumulative incidence of infections at 1 year following CAR-T therapy to be 63% with bacterial infections being most frequent (57%); viral infections(45%); and fungal infections (4%) being less frequent.9
CONCLUSION
Although still uncommon, cutaneous mucormycosis in patients with COVID-19 is a highly serious condition with significant mortality. As such, a high index of suspicion combined with a rapid diagnosis is essential. And when diagnosed, immediate surgical debridement and appropriate anti-fungal medications are crucial.
The authors certify that they have obtained patient’s substitute decision maker’s (SDM) consent, as patient passed away. The SDM gave consent for the images and other clinical information to be reported in the journal. The SDM understands that the patient’s name and initials will not be published, and due efforts will be made to conceal their identity.








