Abstract
Asthma is an obstructive lung disease affecting >230 million people worldwide and a significant cause of morbidity in patients of all ages. It is a heterogeneous disease with a complex pathophysiology and phenotype. Diagnosis is made with thorough history-taking and physical examination, and the condition is characterised by variable airflow obstruction and airway hyper-responsiveness. Understanding the severity of the disease is important, and treatment is aimed at symptom control and the prevention of future exacerbations. Pharmacologic treatment with beta-agonists for intermittent asthma and inhaled corticosteroids and a combination of inhaled corticosteroids and long-acting beta-2 agonists for persistent asthma are recommended. Additional and alternative treatments with leukotriene modifiers, anticholinergics, biologics, and bronchial thermoplasty are also available. However, understanding an individual’s disease phenotype, endotype, and comorbidities is necessary for asthma treatment, with appropriate consultation with asthma specialists required for those with severe asthma.
BACKGROUND
Asthma is a heterogeneous disease that affects many individuals. There are approximately 235 million people worldwide who have asthma,1 and in 2015 there were approximately 383,000 asthma-related deaths.1 In the USA alone, the annual cost of asthma is approximately $56 billion, with a significant proportion of this figure comprising indirect costs, such as days lost from work or school.2 For most patients, asthma can be controlled with appropriate inhaler-based therapy. For many of the more severe asthma patients, significant advances in medical care have reduced exacerbations and improved quality of life. Appropriate diagnosis, recognition of different phenotypes, and an understanding of the various treatment options are paramount in asthma management. This review will focus on the diagnosis and treatment of asthma.
DIAGNOSIS
Asthma is a disease of the lower respiratory tract that affects men and women of all ages. It is diagnosed clinically, but no single gold standard test is available; there is significant heterogeneity to asthma’s pathophysiology and clinical presentation, and clinical overdiagnosis can occur, especially in those without spirometric confirmation.3 Therefore, a thorough history and physical examination along with spirometry are important for the diagnosis of asthma.
Clinical Presentation
The symptoms of asthma can be nonspecific and varied, making the diagnosis difficult. Patients often present with wheezing, shortness of breath, and cough that occur more frequently during the night and early morning.4 Symptoms are often episodic and can be caused by various triggers, such as irritants, specific allergens, and exercise. Wheezing and nocturnal dyspnoea have a strong correlation with diagnosis of asthma (relative risk: 26% and 29%, respectively), and wheezing is the single most sensitive and prevalent symptom for the diagnosis of asthma.5,6 Respiratory symptoms that vary over time and in intensity, that are worse at night or in the morning, and that have specific triggers are associated with a higher likelihood of an asthma diagnosis.7 On the other hand, the presence of chronic sputum production, chest pain, and isolated cough with no other respiratory symptoms decrease the probability of asthma.7 Detailed history-taking is an important step in the diagnosis of asthma and evidence of variable airflow limitation confirmed by a physician is required to confirm the presence of the disease.7
Differential Diagnosis
Asthma can mimic other diseases and, therefore, it is important to consider various differential diagnoses in patients presenting with asthma-like symptoms. Differential diagnoses of asthma include diseases of the upper and lower respiratory tracks, pathologies of the cardiovascular and gastrointestinal systems, and psychiatric conditions (Box 1).8 For example, congestive heart failure can cause wheezing and airflow obstruction from pulmonary oedema and pulmonary vascular congestion, mimicking asthma.9 This condition has been termed ‘cardiac asthma’ and treatment of the underlying heart failure often leads to the improvement of the symptoms.10
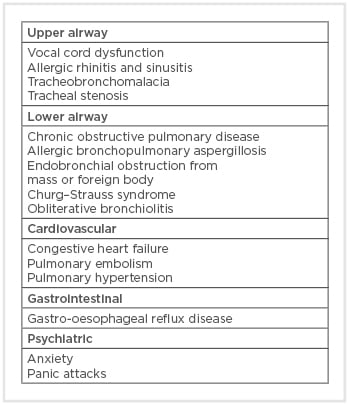
Box 1: Differential diagnoses of asthma.
Vocal cord dysfunction (VCD) is another common differential diagnosis of asthma. These patients often present with recurrent asthma exacerbations that are refractory to corticosteroids or bronchodilator treatment. VCD is caused by episodic extrinsic airway obstruction from paradoxical vocal cord motion and is closely associated with gastro-oesophageal reflux, laryngopharyngeal reflux, anxiety, and depression.11 Recognition of VCD is important in limiting unnecessary corticosteroid exposure and healthcare utilisation.11
Chronic obstructive pulmonary disease (COPD) is a progressive, obstructive lung disease that presents similarly to asthma. Both diseases affect the small airways and have airflow obstruction seen on spirometry; however, COPD patients have limited airway hyper-responsiveness (<12% improvement in forced expiratory flow in 1 second [FEV1] after bronchodilator inhalation on pulmonary function test [PFT]) and often have a significant smoking history. Asthma and COPD can exist as a spectrum of obstructive diseases and can sometimes be difficult to distinguish from one another, especially in patients with chronic, poorly controlled asthma that leads to fixed airflow obstruction due to chronic inflammation and airway remodelling, as this can make the distinction between the two diseases more difficult.4,12 Some of these patients can have chronic persistent airflow obstruction with aspects of asthma and meet the diagnosis for asthma–COPD overlap syndrome.13 Understanding and recognising these two disease processes are important.
Spirometry and Bronchoprovocation Testing
Currently, the Global Initiative for Asthma (GINA) and National Asthma Education Prevention Program (NAEPP) recommend spirometry testing in patients suspected of having asthma.7,14,15 Asthma is characterised by variable airway obstruction and hyper-responsiveness; airflow obstruction with a FEV1/forced vital capacity ratio <0.7 or less than the lower limit of normal (LLN) and airflow reversibility after inhalation of a short-acting beta-2 agonist (SABA) defined as FEV1 improvement by at least 12% and 200 mL indicates a diagnosis of asthma. However, given the variable nature of airflow obstruction asthma patients can present with normal spirometry results.
In such patients with normal spirometry results, bronchoprovocation with methacholine or mannitol can be useful in the asthma diagnosis. A >20% drop in FEV1 provocation concentration (PC20 <16 mg/mL), and now recently a provocation dose (PD20) <400 µg, are currently used and recommended for diagnosis. Methacholine is a direct stimulant of airway smooth muscle by binding to acetylcholine receptors, causing bronchoconstriction and airflow obstruction, and is a sensitive tool for diagnosing asthma. Patients with asthma will have a heightened response to methacholine inhalation, and this test can be used to help diagnose asthma, especially in those with active asthma.16 Mannitol dry powder is an indirect stimulator of bronchoconstriction, which is a more specific than sensitive diagnostic tool. Several studies have shown that both methacholine and mannitol have similar sensitivities and specificities in diagnosing asthma, especially in patients without active symptoms.17,18 Bronchoprovocation testing can therefore be useful in ruling out asthma, especially in patients not currently on inhaled corticosteroid treatment (Figure 1).
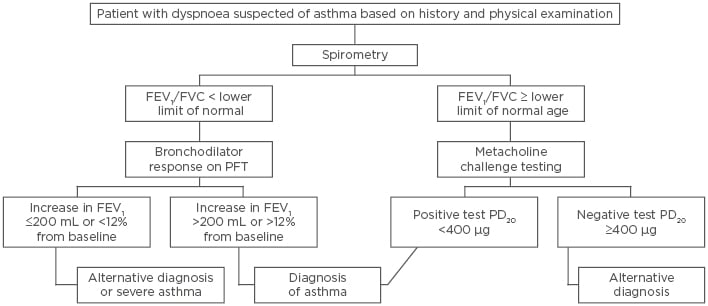
Figure 1: Asthma diagnosis algorithm.
FEV1: forced expiratory volume in 1 second; FVC: functional vital capacity; PC20: provocative concentration causing a 20% decline in FEV1; PD20: provocation dose causing a 20% decline in FEV1; PFT: pulmonary function testing.
Fractional Excretion of Nitric Oxide
Nitric oxide (NO) produced by the airway epithelium is an indirect marker of elevated airway inflammation.19,20 The level of NO in exhaled breath can easily be measured and has been used for detecting airway inflammation in patients suspected of and with the diagnosis of asthma. However, fractional excretion of NO (FeNO) is more sensitive to eosinophilic airway inflammation and is not as useful in the diagnosis of non-eosinophilic asthma.19 The American Thoracic Society (ATS) recommends the use of FeNO measurements <25 ppb in adults to indicate a lower likelihood of eosinophilic inflammation and corticosteroid responsiveness.20
There have been conflicting data regarding the use of FeNO in monitoring asthma. Studies have shown that an elevated FeNO level correlates closely with severity of asthma and that using FeNO and sputum eosinophil count to monitor asthma can help reduce the total exposure to inhaled corticosteroids (ICS).21,22 However, a study by Shaw et al.23 showed that there was no significant reduction in asthma exacerbations or the total amount of ICS use in those monitored using FeNO compared to those not monitored. In addition, a study by Szefler et al.24 showed increased doses of ICS without improved symptoms in those monitored with FeNO testing than those without FeNO monitoring. Despite these findings, the ATS guidelines continue to recommend the use of FeNO measurements in monitoring of disease activity in asthma patients.20,25
Exercise Challenge Testing
For patients with exercise-induced bronchoconstriction (EIB), exercise challenge testing can be used for diagnosis.26 During this test, patients rapidly increase their exercise intensity on a stationary bicycle or treadmill every 2–4 minutes to achieve a high level of ventilation of at least 17.5–21.0-fold their baseline FEV1. A fall in FEV1 >10% meets the diagnosis of EIB, with levels >25% and <50% indicating moderate EIB and >50% indicating severe EIB.26 Exercise challenge testing should be considered in those with a suspicion of EIB with negative work-up.
SEVERITY OF ASTHMA
Understanding the severity of a disease is important for its management. The NAEPP defines severity as the intrinsic intensity of the disease prior to treatment with long-term control therapy;15 an understanding of disease severity to initiate therapy and achieve control of the disease is emphasised in NAEPP guidelines. Asthma severity is divided into intermittent, mild, moderate, and severe and persistent. Frequency of daily symptoms, night-time awakenings, use of SABA, interference with normal life activities, and lung function are all components used to determine disease severity in treatment-naïve asthmatics.
GINA defines asthma severity based on the intensity of active treatment to achieve good control of the disease. Patients need to have been on controller medications for several months to establish severity, with the goal being to titrate treatment to the minimum effective level to maintain control.7 Most patients can achieve good symptom control with long-acting controller medications; however, in patients with persistent symptoms, correct diagnosis, compliance, inhaler technique, comorbid conditions, and ongoing exposure to sensitising agents need to be assessed.
Since asthma is a clinical disease and spirometric measurements do not always reflect a patient’s disease severity, symptom control questionnaires have been developed and validated as a quantitative assessment of patient symptoms. These include the Asthma Control Test (ACT), the Asthma Quality of Life Questionnaire (AQLQ), and the Asthma Control Questionnaire (ACQ), and these can be used at each visit to a doctor to better assess a patient’s symptoms.27-29
In addition, measurements of peak expiratory flow rate (PEFR) are an important objective tool for monitoring a patient’s disease process and intensifying the controller regimen. Studies by Ignacio-Garcia and Gonzalez-Santos30 and Lahdensuo et al.31 showed that subjects with daily PEFR home monitoring as part of an asthma self-management programme had improved healthcare utilisation, a reduction in additional medication use, and an increase in lung function. However, given the significant variation in PEFR measurements, which can be >20% diurnally, GINA currently recommends the use of PEFR monitoring only for patients with severe asthma and those with an impaired perception of significant airflow limitation.7
ASTHMA PATHOPHYSIOLOGY AND ITS PHENOTYPES
Asthma is a chronic airway disease of varying pathophysiology, which includes eosinophilic, neutrophilic, mixed granulocytic, and paucigranulocytic pathways. The classic pathway of asthma involves the release of thymic stromal lymphopoietin by epithelial cells when an allergen or infectious agent enters the airway. This then activates Th2 cells, which produce various cytokines, including IL-4, IL-5, and IL-13. These cytokines then lead to the IgE formation and eosinophil activation responsible for airway hyper-responsiveness (Figure 2).25 Activation of mast cells via the attachment of IgE to high-affinity IgE receptors leads to the release of histamine, cysteinyl leukotrienes, and prostaglandins, which are also involved in bronchoconstriction.32
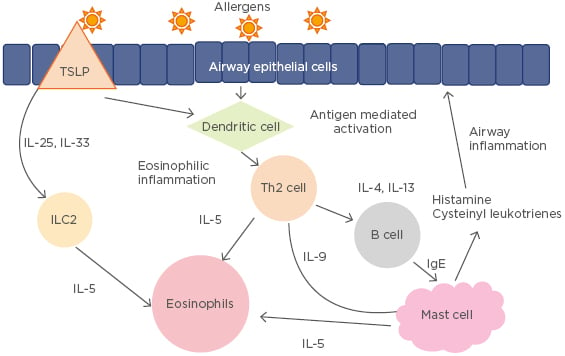
Figure 2: Th2 pathogenesis of asthma.
ILC2: Type 2 innate lymphoid cells; TSLP: thymic stromal lymphopoietin.
The non-eosinophilic pathway of asthma involves activation of airway epithelial cells and macrophages by TLR4 and CD14, which leads to the production of NFκB and IL-8, which further activate neutrophils.33,34 There are many phenotypes and endotypes of asthma, each with a distinct clinical presentation and pathophysiology. Prior large studies have used clinical presentations such as sex, age of onset, allergic status, lung function, and asthma symptoms to categorise asthma patients into clusters. Many different phenotypes have been described but most can be distinguished by early versus late onset, the presence of atopy and significant allergic symptoms, severity of lung function reduction, and response to treatment.32,35,36
The early-onset, allergic phenotypes include those who present with symptoms early in childhood that last into the adulthood. These patients often have elevations in IgE along with associated allergic and atopic symptoms and respond well to treatments that target Th2 response and IgE downregulation. Patients with the late-onset eosinophilic phenotype, on the other hand, present with more severe, persistent symptoms that are less allergic in origin. These patients often do not respond to corticosteroids as well, and their disease process involves predominantly cysteinyl leukotriene pathway upregulation. Eosinophilic phenotype includes patients who exhibit significant sputum eosinophils (>2%) and have good response to corticosteroids. The exercise-induced asthma phenotype involves mast cell and Th2 cytokine activation, often with mild intermittent symptoms that occur during exercise. Patients with the obesity-related phenotype lack Th2 biomarkers and have a less clear pathway to airway hyper-responsiveness. The neutrophilic phenotype includes patients with persistent asthma who are less responsive to corticosteroids. These patients often have elevated neutrophils with exacerbations and tend to respond better to biologics and alternative treatments, including macrolide therapy. Patients with aspirin sensitivity, exercise-induced asthma, and bronchopulmonary mycosis will need additional treatment targeting each non-allergic cause. Therefore, understanding the different phenotypes and endotypes is important in determining one’s treatment course.
As we better understand different asthma phenotypes and the biomarkers that identify them, we can target medical therapy more precisely and develop new agents that target specific pathological pathways of asthma.
TREATMENT
The goal of asthma treatment is symptom control and prevention of future exacerbations.7,8 It involves an understanding of the heterogeneous pathophysiology and phenotypes of asthma and an individualised treatment plan. Patient education and a written asthma action plan can raise awareness of worsening symptoms, impending exacerbations, and the need for titration of therapy for better symptom control.7,8,15 Self-management and a shared care approach have also been shown to improve asthma outcomes.37,38 In addition, education about proper inhaler techniques, medication compliance, and avoidance of allergens and irritants is crucial to all asthma patients.
A stepwise approach to pharmacologic treatment is recommended. The initial choice of medication is determined by the aforementioned asthma severity classification by NAEPP (intermittent, mild, moderate, and severe persistent).15 A step-up or step-down therapy is recommended depending on symptom control based on GINA guidelines.7 Currently, it is recommended that all patients with asthma have SABA inhalers for rescue therapy. In those with persistent asthma, addition of low-dose ICS in titrating doses is recommended. For those with moderate-to-severe persistent asthma, long-acting beta-2 agonists (LABA) or leukotriene inhibitors are often added to the ICS regimen. Select use of biologic agents can be considered for those patients with more severe, difficult-to-control forms of asthma.
Beta-2 Agonists
Beta-2 agonists are bronchodilators that play an important role in asthma control and treatment of acute exacerbations. They bind to the beta-2 adrenergic receptors on the bronchial smooth muscle cells, causing smooth muscle relaxation and bronchodilation.39,40 SABA are often used to treat mild intermittent asthma and acute exacerbations but should not be considered a controller medication; increased use of SABA has been associated with worse asthma control and ICS can sometimes be added to the treatment of those with mild intermittent asthma to limit SABA use.41 SABA are most effective in treating acute bronchoconstriction and have a rapid onset of action of 1–5 minutes, with peak effects at 2 hours and median duration of action of 3 hours.42-44 Examples of SABA include albuterol, levalbuterol, terbutaline, metaproterenol, and pirbuterol.
LABA include salmeterol and formoterol and can have bronchodilatory effects lasting >12 hours.44 However, LABA should only be prescribed in conjunction with ICS in asthma patients. A large randomised control trial (SMART45) investigated >26,000 asthma patients and compared LABA (salmeterol) and placebo when added to usual asthma care. The researchers found that there were more respiratory and asthma-related deaths and life-threatening experiences in those treated with LABA than those receiving placebo.
The safety and benefits of the LABA/ICS combination, however, have been shown in multiple studies. Studies by Peters et al.46 and O’Byrne et al.47 showed that the use of a LABA/ICS combination was associated with a lower risk of asthma exacerbation and improved lung function compared to ICS alone. Therefore, the use of a LABA–ICS combination inhaler is safe and a potential step-up therapy for asthma patients.
Corticosteroids
Corticosteroids are integral to the management of acute asthma exacerbations and chronic disease control because a significant portion of asthmatic patients have an inflammatory phenotype. ICS are an important part of persistent asthma management, especially for those patients with an eosinophilic phenotype. The drugs decrease airway hyper- responsiveness and inflammatory response to allergens by downregulating eosinophil and mast cell activation.48 Studies have shown that the use of ICS (budesonide) improved peak flow measurements in asthma patients compared to those on beta-agonist treatment only.49,50 ICS have also been shown to reduce the rates of exacerbations and improve lung function.51,52 In patients with moderate-to-severe persistent asthma, the addition of LABA to ICS has been found to be beneficial. Studies by Kavuru et al.53 and Shapiro et al.54 showed that a combination of salmeterol and fluticasone resulted in improvements in PEFR, reduced symptom scores, nocturnal symptoms, and albuterol use compared to fluticasone alone. A study by O’Byrne et al.47 showed that ICS alone reduced the risk of severe exacerbations and poorly controlled symptom days, and that the addition of LABA to ICS further improved overall lung function. Examples of currently available ICS include beclomethasone, triamcinolone, flunisolide, ciclesonide, budesonide, fluticasone, and mometasone.
Systemic corticosteroids are especially important in the treatment of uncontrolled asthma and acute asthma exacerbations. Short-term use of systemic corticosteroids can be an effective tool in decreasing systemic inflammation and bronchial constriction. However, long-term use of systemic corticosteroids is discouraged due to their association with numerous long-term side effects, including weight gain, gastritis, osteoporosis, hypertension, adrenal suppression, and psychosis. There is no standard recommended duration or dosage of corticosteroids for acute asthma exacerbation treatment.55 Patients who are unable to be weaned from systemic corticosteroids to maintain disease control should be assessed for treatment with biologic medications and for comorbid conditions and referred to an asthma specialist.
Leukotriene Receptor Antagonists and Synthesis Inhibitor
Leukotrienes are lipid mediators involved in bronchoconstriction and airway inflammation. Leukotriene-modifying drugs, including zafirlukast, montelukast, and zileuton, work by inhibiting leukotriene synthesis or as competitive antagonists of the leukotriene receptors.45 Cysteinyl leukotrienes are released from mast cells and eosinophils and are involved in bronchial smooth muscle contraction and increased mucus secretion.56 By working as receptor antagonists and inhibiting leukotriene synthesis, these drugs downregulate airway inflammation; they have also been shown to improve asthma symptoms and lung function and serve as an add-on therapy to ICS. Current guidelines recommend the use of leukotriene receptor antagonists only as an alternative treatment to ICS in those with moderate persistent asthma who cannot tolerate ICS and as an add-on therapy to those receiving combined LABA/ICS.
Antimuscarinics
The use of antimuscarinics for alleviating bronchoconstriction and dyspnoea dates back hundreds of years.57 The parasympathetic system, controlled by acetylcholine and the activation of muscarinic receptors, contributes to airway smooth muscle constriction and mucous secretion.58 Antimuscarinics are used to disrupt this vagally mediated muscarinic receptor activation, leading to subsequent bronchodilation. Currently available short-acting muscarinic antagonists (SAMA) include ipratropium and long-acting muscarinic antagonists (LAMA) include tiotropium, aclidinium, umeclidinium, and glycopyrronium.
Both SAMA and LAMA can be used to treat severe, poorly controlled asthma exacerbations and as an add-on maintenance therapy to LABA/ICS therapy.59 Peters et al.60 studied the effectiveness of the addition of tiotropium to beclomethasone compared to adding salmeterol to beclomethasone or doubling of the beclomethasone dose in 210 asthma patients. The results showed that the addition of tiotropium had greater improvements in PEFR, asthma control days, FEV1, and daily symptoms compared to the doubling of ICS or addition of salmeterol.60 In addition, two replicate trials, PrimoTinA-asthma 161 and PrimoTinA-asthma 2,62 investigated the effectiveness of tiotropium in patients with poorly controlled asthma on high-dose ICS/LABA treatment. This study showed that those who received additional tiotropium had an improved FEV1 and time to first severe exacerbation, and a 21% reduction in exacerbation risk.63 LAMA remain a potential treatment for those with poorly controlled asthma.
Biologic Therapy
For those with severe asthma, the use of biologic agents should be considered carefully. Targeted use of biologic therapy allows these patients to achieve control while limiting their oral corticosteroid exposure (Table 1).64-72 Omalizumab is the first approved biologic for asthma and works by binding to IgE and downregulating activation of airway inflammation. In clinical trials, omalizumab has been shown to reduce overall asthma exacerbation rates by 25% and severe exacerbations by 50%, as well as improving asthma quality of life in those with uncontrolled moderate-to-severe asthma with perennial aeroallergen sensitivity.64
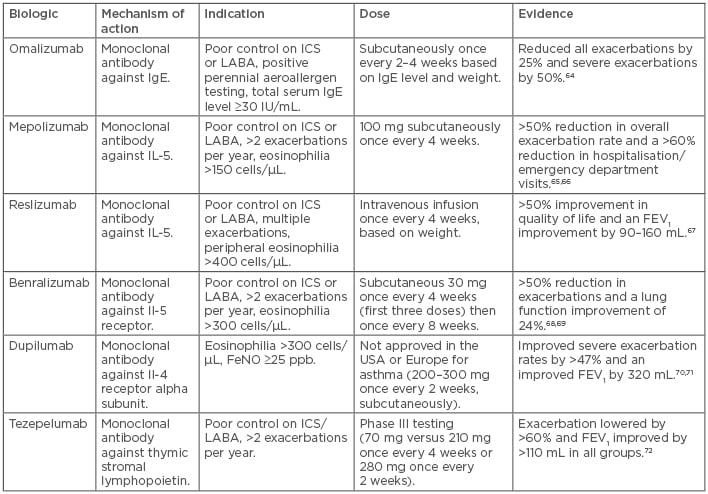
Table 1: Biologics for asthma treatment.
FEV1: forced expiratory volume in 1 second; FeNO: fractional excretion of nitric oxide; ICS: inhaled corticosteroids; LABA: long-acting beta-2 agonists.
Newer biologic agents targeting IL-5 pathways are also available. IL-5 is a major cytokine responsible for the growth, differentiation, and survival of eosinophils, which play a large role in airway inflammation. Mepolizumab is a humanised monoclonal antibody against IL-5, hence it blocks the IL-5 pathway. Mepolizumab trials have shown a >50% reduction in overall exacerbation rate, >60% reduction in hospitalisation or emergency room visitation rates, improvements in quality of life scores, and a 50% reduction of oral corticosteroid dose for those who are on chronic oral corticosteroids.65,66
Reslizumab is another monoclonal antibody against IL-5 that is approved for use in those with poorly controlled asthma and with IgE levels ≥400 cells/uL. Clinical trials have shown an improved exacerbation rate by >50%, increased asthma quality of life, and improved lung function by 90–160 mL over placebo, especially in those with higher levels of peripheral eosinophils.67 Benralizumab is also a monoclonal antibody against IL-5 receptor that causes the body’s own natural killer cells to target and eliminate eosinophils. It has been shown to reduce exacerbations by >50%, reduce the dose of chronic oral corticosteroids use by 75%, and improve lung function by 24%.68,69
Other biologics include dupilumab, a monoclonal antibody against IL-4 receptor that blocks IL-4 and IL-13. From Phase III trial data, dupilumab has been shown to reduce exacerbations, improve lung function, and reduce chronic oral corticosteroid use.70,71 It is particularly more effective in patients with peripheral eosinophil levels >300 cells/µL and FeNO levels ≥25 ppb. Tezepelumab is a monoclonal antibody that blocks the action of the cell signalling protein thymic stromal lymphopoietin and downregulates the inflammatory pathway responsible for asthma. This drug is currently undergoing Phase III studies but has shown a significant decrease in asthma exacerbation rates in a Phase II study.72 As more biologics become available, phenotyping and endotyping of each patient are necessary to provide insights into the most appropriate long-term therapy.
Bronchial Thermoplasty
Bronchial thermoplasty (BT) offers a non-pharmacologic therapy for those with asthma unresponsive to standard treatment with ICS and bronchodilators. BT uses thermal energy to bronchoscopically ablate airway smooth muscles to decrease bronchoconstriction and airway hyperplasia.73 The effectiveness of this treatment was initially seen in the AIR trial in 2007, which randomised patients with moderate or severe asthma to BT or a control group. Those who received BT had significant improvements in morning PEFR, percentage of symptom-free days, and symptom score reduction.74 In addition, the RISA trial randomised 32 poorly controlled asthma patients to BT or a control group and reported that the BT group had increased initial short-term morbidity but significantly improved pre-bronchodilator FEV1 and asthma symptom scores.75 These studies were followed by the AIR2 trial, which again demonstrated significant improvements in asthma symptoms and exacerbations in those randomised to BT.74 BT may therefore be an effective non-pharmacologic treatment for asthma in those with severe disease resistant to pharmacotherapy; however, there are significant adverse reactions associated with BT, including life-threatening severe exacerbations and death.74,75
COMORBID CONDITIONS
Treatment of comorbid conditions and avoidance of environmental and allergic triggers are important in asthma management. For example, obesity, gastro-oesophageal reflux disease, anxiety and depression, rhinitis and sinusitis, and seasonal and perennial allergies have all been associated with worsening asthma symptoms.8,15,76-79 Additional treatments targeting these comorbidities can significantly improve asthma control, especially in those with severe asthma.
CONCLUSION
Asthma is a heterogeneous disease affecting millions of people worldwide. It is characterised by airway hyper-responsiveness and airway inflammation with variable airflow obstruction. Understanding the various phenotypes and pathophysiologies and providing individualised treatment that is suited to the patient’s comorbidities and lifestyle is important in the management of asthma.







