Abstract
Surgery provides the best chance of a cure for patients with early stage non-small cell lung cancer (NSCLC) and plays an important role in the multimodal treatment for locally advanced disease. However, many patients still relapse despite intended curative surgery, and no major advances in systemic therapy for resectable NSCLC have been achieved in the last decades. The incorporation of immunotherapy for the treatment of metastatic Stage IV NSCLC and the recent data on the efficacy of cancer consolidation with the anti-programmed death-ligand 1 (PD-L1) antibody durvalumab after concurrent chemoradiation for unresectable Stage III NSCLC open new opportunities for the use of immune checkpoint inhibitors in earlier stages of the disease. Multiple ongoing clinical trials are exploring the safety and efficacy of immunotherapy in Stage I–III resectable NSCLC, either as a postoperative (adjuvant) strategy or before surgical resection (neoadjuvant). The neoadjuvant setting is particularly interesting, as it represents an ideal chance to develop translational research.
Herein, the authors summarise the main ongoing research and available data on the use of anti-PD1/PD-L1 antibodies for Stage I–III NSCLC.
INTRODUCTION
Non-small cell lung cancer (NSCLC) is the leading cause of cancer mortality worldwide.1 Survival strongly depends on the stage of disease at presentation, which was recently revised for the eighth edition of the tumour, node, and metastasis (TNM) classification for lung cancer.2 According to staging, early NSCLC is defined as Stage I–II, while locally advanced (non-metastatic) disease refers to Stage III, which represents a highly heterogeneous group of patients with different tumour extent and controversial management based on the combination of surgery, chemotherapy (CT), and radiotherapy (RT).
Surgery offers the best chance of survival for patients with early stage and locally advanced resectable NSCLC. However, 5-year survival rates decline from 77–92% for Stage IA patients to 36% for Stage IIIA patients, with many patients experiencing disease relapse during postoperative follow-up.3
The use of immune checkpoint inhibitors that reverse cancer immunotolerance and enhance antitumour immune response has demonstrated durable remissions for a subset of patients with metastatic Stage IV NSCLC, in both the first4-10 and second-line setting in recent years,11-14 with a manageable safety profile. These immune checkpoint inhibitors include monoclonal antibodies directed against programmed cell death protein 1 (PD1) (nivolumab, pembrolizumab), PD-ligand 1 (PD-L1) (atezolizumab, avelumab, durvalumab), and the cytotoxic T lymphocyte- associated antigen-4 (CTLA-4) (ipilimumab, tremelimumab). The Phase III PACIFIC trial15 comparing durvalumab versus placebo following concurrent chemoradiation in patients with unresectable Stage III NSCLC showed a significant improvement in progression-free survival (PFS) with the anti-PD-L1 antibody as consolidation therapy. The improvement was consistent across all patient subgroups that were analysed, irrespective of baseline expression of PD-L1 on tumour cells. Durvalumab was reasonably well tolerated with a manageable safety profile.15 Based on PFS data, in February 2018, the U.S. Food and Drug Administration (FDA) approved durvalumab for the treatment of patients with locally advanced, unresectable, Stage III NSCLC who have not progressed following chemoradiation therapy. A statistically significant overall survival benefit was also determined by an independent data monitoring panel at an interim analysis in May 2018,16 providing additional evidence of the clinical benefit that durvalumab can offer NSCLC patients in this setting. The use of durvalumab after chemoradiation is a new standard of care for unresectable Stage III NSCLC patients. After concurrent therapy, this has been the first major advance in Stage III NSCLC in the past decade.
Based on the efficacy of immunotherapy (IT) in advanced NSCLC, earlier stage disease is the next frontier for immune checkpoint inhibitors. Multiple clinical trials are currently ongoing to test their role in Stage I–III resectable NSCLC, either as postoperative (adjuvant) strategy or preoperative resection (neoadjuvant). In this review, the authors summarise the key ongoing research and available data on the use of anti-PD1/PD-L1 antibodies for Stage I–III NSCLC.
ANTI-PROGRAMMED CELL DEATH PROTEIN 1/PROGRAMMED CELL DEATH PROTEIN LIGAND 1 IMMUNOTHERAPY IN INOPERABLE STAGE I–II NON-SMALL CELL LUNG CANCER
Surgery is the standard of care for Stage I–II NSCLC patients who are medically fit. Stereotactic body radiation therapy (SBRT) can be an alternative strategy for those patients who are medically inoperable or refuse surgery.17,18 The efficacy of IT is being investigated across studies in this setting. A currently ongoing Phase I trial is evaluating atezolizumab in combination with SBRT in patients with inoperable Stage I NSCLC.19 Another Phase I/II trial for patients with Stage I NSCLC who have been deemed medically inoperable or who have refused surgery is also testing the safety and tolerability of definitive SBRT combined with concurrent and adjuvant avelumab.19
The concurrent use of anti-PD1/PD-L1 antibodies with RT has the potential benefit of boosting the abscopal effect, a phenomenon in the treatment of metastatic tumours where localised radiation triggers systemic antitumour effects outside the scope of the localised treatment.20 Abscopal effects after SBRT have been reported in both renal cell carcinoma and melanoma.21-23 For patients unsuitable for surgery with early stage NSCLC, nivolumab is going to be tested in the Phase II STILE trial24 on completion of SBRT, although this trial is not yet recruiting.
ANTI-PROGRAMMED CELL DEATH PROTEIN 1/PROGRAMMED CELL DEATH PROTEIN LIGAND 1 IMMUNOTHERAPY AS ADJUVANT TREATMENT AFTER SURGERY IN STAGE I–III NON-SMALL CELL LUNG CANCER
In patients with completely resected Stage II or IIIA NSCLC, adjuvant platinum-based CT improves survival and is currently considered the standard of care.25 In contrast, no significant improvement of adjuvant CT has been observed for resected Stage I NSCLC, although results from a subgroup analysis indicate that it would be reasonable to consider adjuvant CT for larger tumours (≥4 cm).25 The use of adjuvant RT after successful R0 resection is still under debate, but recent studies have demonstrated clinical benefit in patients with N2 nodal stage diagnosed surgically.26,27 Particularly in patients with clinically unforeseen N2 disease that have undergone complete resection, adjuvant CT and RT are still insufficient to provide optimal outcomes. Several clinical trials are currently recruiting patients with completely resected NSCLC to assess the efficacy of adjuvant anti-PD1/ PD-L1 antibodies. Many of these trials allow for the combination of immune checkpoint inhibitors with adjuvant CT and/or RT (Table 1).28-33
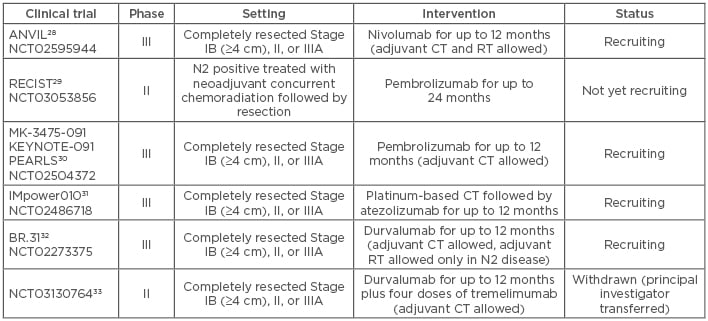
Table 1: Clinical trials of adjuvant anti-programmed death receptor 1/programmed death-ligand 1 immunotherapy in non-small cell lung cancer.
CT: chemotherapy; RT: radiotherapy.
One possible caveat for the adjuvant use of IT is that optimal lung cancer surgery requires mediastinal lymphadenectomy while tumour-associated lymphatic vessels and lymph nodes could play an important role in antitumour immune response regulation.34,35 Neoadjuvant use of IT might circumvent this problem.
ANTI-PROGRAMMED CELL DEATH PROTEIN 1/PROGRAMMED CELL DEATH PROTEIN LIGAND 1 IMMUNOTHERAPY AS NEOADJUVANT TREATMENT IN RESECTABLE NON-SMALL CELL LUNG CANCER
Preoperative CT (also referred to as induction or neoadjuvant CT) has proven as effective and better tolerated than postoperative (adjuvant) CT in resectable NSCLC.36,37 Neoadjuvant CT has the potential to reduce tumour size, eradicate micrometastasis, and improve patient compliance with treatment compared to adjuvant CT. In general, patients with Stage IB, II, or IIIA resectable NSCLC may be treated with neoadjuvant CT before surgery.38 So far, there is little data on the efficacy of IT for resectable NSCLC and it is unknown whether the benefit is greater as a neoadjuvant or adjuvant strategy.
Based on mice models of breast cancer, neoadjuvant IT demonstrated a greater efficacy compared with adjuvant IT in preventing metastasis following primary tumour resection.39 In this preclinical study, the authors evaluated and identified sustained peripheral tumour-specific immune responses that underpinned the outcome.
Patients with early stages of NSCLC may have a more intact immune system and reduced tumour conal heterogeneity compared to advanced disease, leaving a window of opportunity for immune checkpoint inhibitors to enhance systemic host immunity.40 The presence of a tumour in the neoadjuvant setting is important as a source of neoantigen to prime the immune response, and neoadjuvant IT may contribute to T cell activation and expansion before the primary tumour is surgically removed, which may ultimately lead to an effective long-term systemic surveillance of micrometastasis. The neoadjuvant setting is particularly interesting too, as it allows identification of the effects of IT on pathologic tumour response.
In April 2018, Forde et al.41 published the results from a pilot study evaluating two neoadjuvant doses of the anti-PD1 antibody nivolumab in untreated resectable (Stage I, II, or IIIA) NSCLC. This study demonstrated that preoperative PD1 blockade was feasible, had acceptable side effects, did not delay planned surgery, and induced a major pathologic response in 45% of resected tumours, even when preoperative radiologic imaging failed to demonstrate tumour shrinkage. Tumour mutation burden correlated significantly with pathologic response, and neoadjuvant treatment induced expansion of tumour-specific T cell clones in peripheral blood.41
The association between pathologic response to neoadjuvant CT and survival in resectable NSCLC has been addressed in some retrospective studies.42,43 As PFS and overall survival are long-term endpoints for early- stage lung cancer trials and it takes years for data to mature, future studies are needed to validate pathologic response after neoadjuvant therapies as a surrogate endpoint for survival.
Currently, neoadjuvant IT for resectable NSCLC is under investigation in several clinical trials and data from these trials are eagerly awaited (Table 2).44-59 The Phase II NADIM trial evaluating preoperative nivolumab plus CT for resectable Stage IIIA NSCLC, followed by adjuvant nivolumab after surgery, conducted by the Spanish Lung Cancer Group, reported its first results in June 2018 at the American Society of Clinical Oncology (ASCO) Annual Meeting.44 Forty-three patients were enrolled in the trial and 22 underwent surgery. Thirteen cases (59%) achieved a complete pathologic response, four cases (18%) had a major pathologic response (defined as <10% viable tumour cells in the resection specimen), and five cases (23%) had a partial response.60
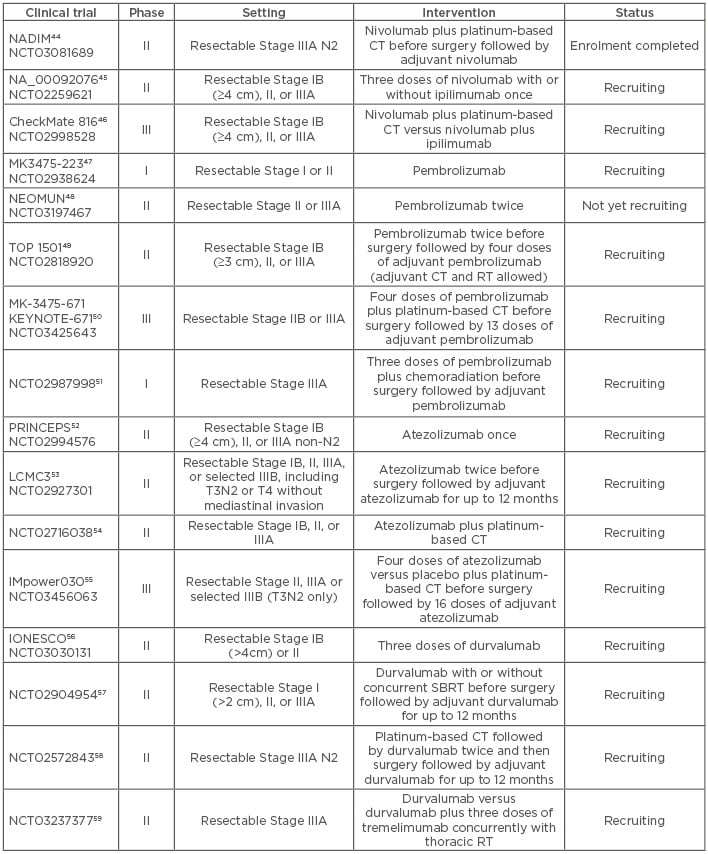
Table 2: Clinical trials of neoadjuvant anti-programmed death receptor 1/programmed death-ligand 1 immunotherapy in non-small cell lung cancer.
CT: chemotherapy; RT: radiotherapy; SBRT: stereotactic body radiotherapy.
DISCUSSION
The benefit of immune checkpoint blockade in early stage and resectable, locally advanced NSCLC remains unknown. Several clinical trials are underway exploring the role of adjuvant and neoadjuvant IT in this setting. Although preclinical data in breast cancer mice models suggest that neoadjuvant IT strategies have a better efficacy than adjuvant IT strategies in preventing metastases after primary tumour resection, the optimal approach in resectable NSCLC needs to be confirmed.
In the neoadjuvant setting, some published data41 may change future clinical practice in non-metastatic NSCLC. Pathologic response to neoadjuvant IT may serve as a surrogate endpoint for predicting survival in resected NSCLC, but further validation is warranted.
Until now, the expression of PD-L1 assessed by immunohistochemistry is the commonly used biomarker for selecting NSCLC patients most likely to benefit from immune checkpoint inhibitors. However, tumour responses to anti-PD1/PD-L1 antibodies have also been described in patients with PD-L1-negative tumours, suggesting that other factors related to tumour microenvironment beyond PD-L1 expression may determine the response to IT.61 Tumour mutational burden and IFN-γ, among others, are emerging biomarkers of response to immune checkpoint blockade62,63 and immune-related gene expression signatures are under development. The ongoing trials on the neoadjuvant use of anti-PD1/PD-L1 antibodies for resectable NSCLC patients may facilitate a comprehensive exploratory characterisation of the tumour microenvironment and the changes in tissue-based biomarkers that might correlate with pathologic response.
Substantial efforts are also needed to address many questions regarding the use of immune checkpoint inhibitors in Stage I–III NSCLC, such as the ideal timing of IT in relation to surgery, method of CT and RT delivery, the optimal dose, schedule, and duration of therapy. The PACIFIC trial16 evaluating durvalumab after chemoradiation in unresectable Stage III NSCLC showed benefit, even though only 40% of the patients assigned to the intervention arm completed the 12 months of planned therapy, suggesting that perhaps optimal duration of therapy could be <1 year. The NADIM trial,44 which evaluated preoperative IT with CT for resectable NSCLC, used three cycles of nivolumab before surgical treatment. The current trials focussing on combining checkpoint inhibitors with CT and RT are expected to help to better define optimal schedules.
CONCLUSION
In view of the efficacy results of IT in metastatic and unresectable NSCLC, earlier stage disease is the next frontier for immune checkpoint therapies. A number of clinical trials are currently investigating the efficacy and safety of anti-PD1/PD-L1 antibodies in Stage I, II, and resectable III NSCLC, either as adjuvant or neoadjuvant strategy, alone or in combination with CT and/or RT. Data from these trials are eagerly awaited while optimal endpoints are being defined.








