BACKGROUND AND AIMS
Sleep disorders are characterized by the impairment of the quality, timing, and amount of sleep, which results in daytime distress and functioning. Primary ciliary dyskinesia (PCD) is a rare genetic condition characterized by oto-sino-pulmonary pathologic manifestations with multiple comorbidities, including sleep disorders. The available literature on sleep disorders and their neuropsychiatric comorbidities in PCD is limited, especially for Hispanic patients.1,2 This pilot study aims to assess the presence of sleep disorders and neuropsychiatric comorbidities in patients with the RSPH4A PCD founder mutation (c.921+3_921+6delAAGT) in Puerto Rico.
The aims of this study were to identify sleep-related disorders in PCD patients with the Puerto Rican founder mutation, and assess their neuropsychiatric manifestations. The researchers also aimed to recognize the importance of early diagnosis and prevention of PCD-related obstructive sleep apnea (OSA) or other sleep-related disorders in pediatric and adult patients with PCD.3
MATERIALS AND METHODS
The authors performed a retrospective case series which included 15 patients with PCD (n=15; 10 pediatric and 5 adult), at the Puerto Rico PCD Foundation. Sleep questionnaire reports were obtained by the pediatric modified STOP-Bang (PM-STOP-Bang) and standardized (STOP-Bang) criteria for pediatric and adult patients, respectively.4 These tools aid in identifying risk factors for respiratory obstruction and neuropsychiatric manifestations. A STOP-Bang Score of >3 points correlates to a moderate risk of OSA in children and adults. In addition, sleep-behavioral manifestations in the areas of cognition and mood were assessed.
RESULTS
Most pediatric patients presented with a PM-STOP-Bang >3 (90%), for a moderate-to-high risk (Figure 1). Obstructive risk factors such as snoring (70%), tonsillar hypertrophy (50%), nasal polyps (50%), and bronchiectasis (70%) were identified (Figure 2). The most common neuropsychiatric symptoms reported were poor concentration (70%), oppositional behaviors (50%), irritability (30%), and hyperactivity (20%) (Figure 1). Furthermore, the adult population showed a STOP-Bang >5 (60%), with high-risk. Obstructive risk factors included snoring (60%), BMI >35 kg/m2 (40%), and bronchiectasis (100%; Figure 3). Neuropsychiatric presentation with tiredness (80%), poor concentration (40%), and irritability (20%) was reported (Figure 4).
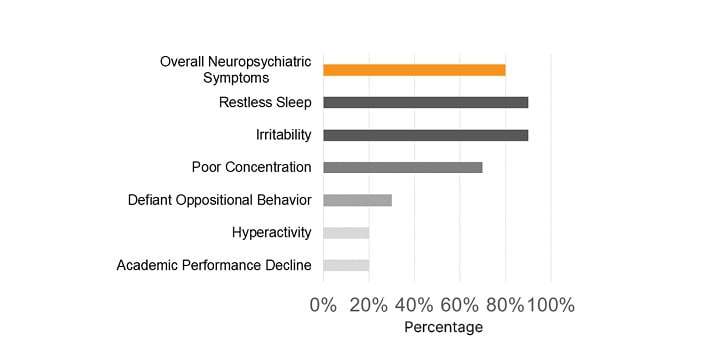
Figure 1: Most common sleep-related neuropsychiatric symptoms were reported in pediatric patients with primary ciliary dyskinesia.
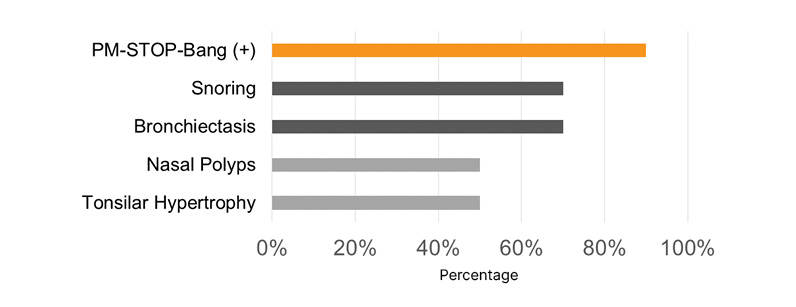
Figure 2: Risk factors related to sleep-related disorders in pediatric patients with primary ciliary dyskinesia.
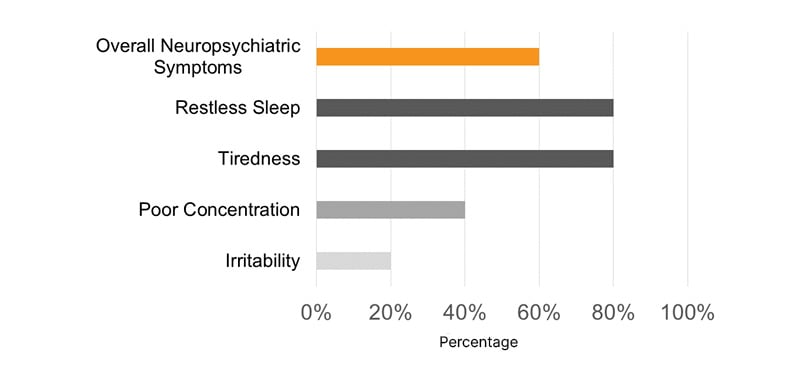
Figure 3: Most common sleep-related neuropsychiatric symptoms were reported in adult patients with primary ciliary dyskinesia.
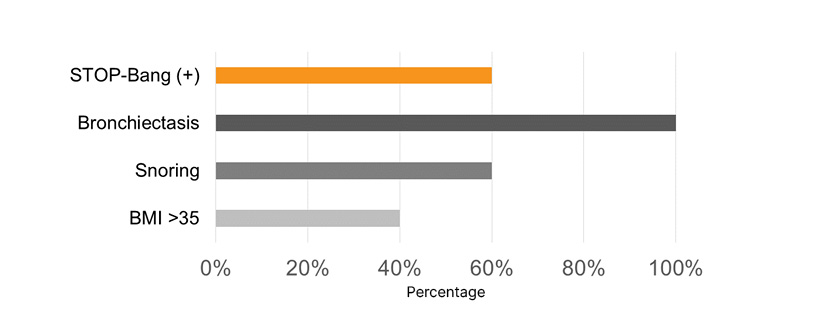
Figure 4: Risk factors related to sleep-related disorders in adult patients with primary ciliary dyskinesia.
Conclusion
Based on the data, most PCD patients with the RSPH4A (c.921+3_921+6delAAGT) PCD founder mutation presented with a high risk for OSA, and neuropsychiatric manifestations as seen in patients with this sleep disorder. This observation would warrant further studies, including physical examination, screening protocols, sleep studies, and neuropsychological testing. Additional studies are required in multicentric clinical trials to allow an understanding of whether these neuropsychiatric manifestations are related to a neurodevelopmental or neurodegenerative process due to this rare ciliopathy.3,5,6








