BACKGROUND AND AIMS
Angioedema can present as a benign symptom or a life-threatening emergency. Angioedema accompanied by anaphylaxis is generally caused by allergic reactions due to IgE-mediated hypersensitivity that leads to mast cell degranulation.1 Melatonin is an unregulated supplement that providers commonly prescribe for its effect on sleep onset and maintenance.2 Here, the authors present a patient who developed angioedema and anaphylaxis after melatonin ingestion.
MATERIALS AND METHODS
A 68-year-old male without a history of allergy, eosinophilia, or atopy administered melatonin 2 nights prior to presentation, and woke up with minor facial swelling, which resolved shortly after. They used melatonin again the following night, and quickly awoke with acute dyspnea and worsened facial swelling. They were given intramuscular diphenhydramine en route to the emergency department, but presented with hypotension and an oral cavity obstructed by glossal edema (Figure 1A).
RESULTS
Laboratory results revealed peripheral eosinophilia, while imaging did not reveal pertinent findings. They were administered intravenous methylprednisolone and intramuscular epinephrine, and subsequently admitted to a progressive care unit. Complement 4 was normal, reducing the likelihood of complement 1 esterase deficiency. The patient did not report prior melatonin, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker use, and family history of angioedema. Furthermore, a thorough medication review did not reveal any significant drug-drug interactions with melatonin. Laryngoscopy revealed no airway compromise. The following day, re-examination revealed significantly improved facial and tongue edema with increased oral cavity space (Figure 1B). The patient was at their baseline 2 days later, and was discharged with a 5-day prednisone course and an EpiPen® (Viatris, Canonsburg, Pennslyvania, USA). The patient did not complete outpatient allergy testing as they chose instead to avoid melatonin. Upon 1-year follow-up, the patient denied any further episodes of angioedema.
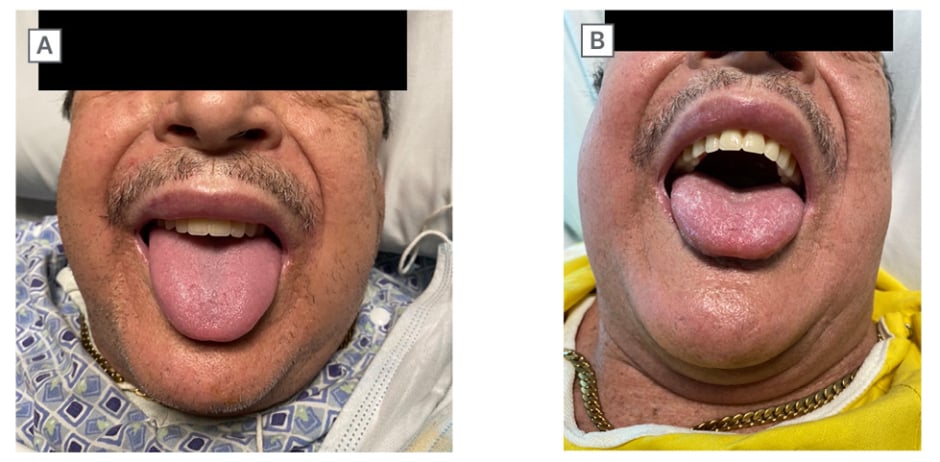
Figure 1: Patient images of improved angioedema following medical management.
A) Patient showing facial and glossal edema. B) Improved angioedema following medical management.
CONCLUSION
Although relatively well tolerated, melatonin supplements have rarely been reported to cause angioedema with symptoms of flushing, difficulty breathing, and dysphagia.3 To the authors’ knowledge, this is the first case of melatonin-induced anaphylaxis. As melatonin is a naturally produced hormone, the allergic agent in this case is likely an unregulated excipient. A previous study found that among 31 different commercial melatonin supplements, the melatonin percentage of the labelled content ranged from -83–+478%.4 Compared with prescription drugs, dietary supplements are regulated less strictly by the U.S. Food and Drug Administration (FDA), and may contain unlabeled, potentially allergenic, inactive ingredients.5 Therefore, a comprehensive medication review that includes over-the-counter drugs and dietary supplements is necessary to identify an etiology for new onset allergic symptoms, in this case as severe as angioedema and anaphylaxis.








