INTRODUCTION
Long-term macrolide therapy is a treatment option in chronic obstructive pulmonary disease (COPD) patients with frequent exacerbations. Long-term azithromycin therapy in COPD patients can reduce COPD exacerbation frequency and improve quality of life.1,2 However, treatment response is heterogenous and the modulation of inflammation by macrolides is not fully understood.
CASE SERIES
After an exceptional clinical response to macrolide therapy in a patient with COPD at our centre, it was noted that the peripheral blood eosinophil count (PBE) increased steadily while on macrolide therapy. Sputum mediator analysis using Luminex platforms (Luminex Corporation, Chicago, Illinois, USA) was performed on the same patient’s sputum samples before, during, and after azithromycin therapy (Figure 1). During azithromycin therapy, interleukin (IL)-8, IL-17A, thymic stromal lymphopoietin, and tumour necrosis factor-alpha decreased, IL-5 and IL-33 increased, and there was not much change in IL-4 and IL-13 levels during azithromycin therapy.
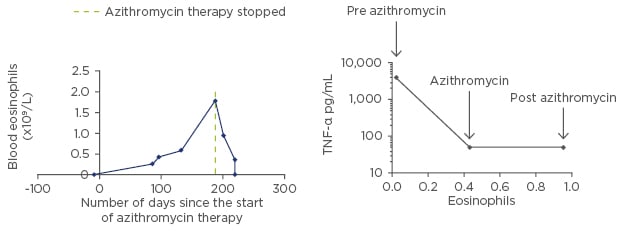
Figure 1: The results of the sputum mediator analysis using Luminex platforms performed on the index patient’s sputum samples from before, during, and after azithromycin therapy.
TNF-α: tumour necrosis factor-alpha.
A retrospective case series review of the effect of long-term macrolide therapy on PBE levels in COPD patients was performed. All COPD patients attending the COPD clinic at our Trust who started long-term macrolide therapy (azithromycin) in 2016 were included (n=16), and anonymised data was audited. The dose of azithromycin used was 250 mg three times a week (n=15) and 250 mg daily (n=1). The overall mean PBE increased during azithromycin therapy (mean change in mean PBE was 0.05; standard deviation: 0.18). There was no significant difference between sex (p=0.51), mean age (p=0.44), smoking status (p=0.66), and mean forced expiratory volume in the 1st second at the start of azithromycin therapy (p=0.55) between patients who had an overall increase in PBE and in those who had an overall decrease in PBE, although numbers were small. There was a reduction in the mean COPD exacerbation frequency in patients with a mean rise in PBE during azithromycin therapy (decreased from 15.5 to 2.1 exacerbations per year). In the group of patients who had a mean decrease in PBE, there were inadequate data available on exacerbations.
DISCUSSION
COPD is typically associated with T helper 1 inflammation with a neutrophilic response, while asthma is characterised by T helper 2 (T2) inflammation,3 although eosinophilic inflammation in COPD is recognised.4 Eosinophilic inflammation is mainly propagated by T2 cytokines such as IL-4, IL-5, and IL-13. In the index case, during azithromycin therapy, IL-8, IL-17A, thymic stromal lymphopoietin, and tumour necrosis factor-alpha decreased, reflecting an overall decrease in the inflammatory burden. IL-5 and IL-33 increased during therapy, which was expected given the associated rise in eosinophils, and there was not much change in IL-4 and IL-13 levels during azithromycin therapy, although the baseline levels were elevated, possibly reflecting an alternative mechanism for T2 inflammation in COPD.
In COPD, a PBE <2% is associated with an increased risk of developing pneumonia independent of inhaled corticosteroid use,5 and COPD patients with a PBE ≥2% have greater improvements in their health-related quality of life and faster recovery after receiving oral corticosteroids during an exacerbation, whilst inhaled corticosteroids decrease exacerbations at stable state.6
COPD is heterogeneous and inflammation can be variable.3 Long-term azithromycin may affect the underlying inflammatory COPD phenotypes, suppressing the T helper 1 neutrophilic inflammation, with an increase in the T2 eosinophilic inflammation, possibly indicating more steroid-responsive disease; however, larger studies are required to further investigate this.








