Meeting Summary
In celebration of the 40th anniversary of the first in vitro fertilisation (IVF) baby this year, the symposium focussed on the modern-day approach to ovarian stimulation (OS). Chairperson Prof Fauser welcomed delegates with a look at the key achievements related to OS in the context of assisted reproductive technologies (ART) over the past century. Treatments have evolved from the first crude preparations to the refined gonadotrophin products available for clinical use today.
The theme of personalisation in OS was introduced by Dr Labarta, who looked at how we can use accurate biomarker measurements to assess ovarian reserve, predict ovarian response, and, therefore, personalise treatment accordingly. Of the biomarkers currently available, anti-Müllerian hormone (AMH) has been identified as the best tool for individualised gonadotrophin dosing. AMH can also be used to drive evidence-based decisions in the choice of gonadotrophin treatment. Dr Alper presented results from the MEGASET HR trial, which investigated highly purified human menopausal gonadotrophin (HP-hMG) in patients identified via their AMH levels as potential high responders. Dr Havelock then demonstrated how AMH, along with body weight, has allowed for the development of the first dosing algorithm for tailoring treatment with follitropin delta, which has been validated in randomised controlled trials (RCT). Finally, the symposium closed with Prof Fauser concluding that, using the biomarker AMH, it is now possible to personalise not only the dose of gonadotrophin but also the choice of gonadotrophin treatment, representing important first steps in truly individualising OS.
Evolution of Gonadotrophin Preparations for Ovarian Stimulation
Professor Bart Fauser
This year marks the 40th anniversary of the first IVF baby, and this milestone is a clear reminder of just how much ART has evolved. OS in particular has come a long way since the early 20th century: development of the first gonadotrophin preparations began in 1910 and has since advanced to the present-day profile of gonadotrophin products (Figure 1).1-6 Today, clinicians have multiple gonadotrophin preparations in their armamentarium, including follicle-stimulating hormone (FSH), luteinising hormone, and human chorionic gonadotropin. All three gonadotrophins are structurally comparable, each containing an identical alpha subunit along with a unique beta subunit, while being subject to specific and individual post-translational modifications.7 Glycosylation is one such modification that can result in the formation of multiple isoforms of each gonadotrophin.8,9
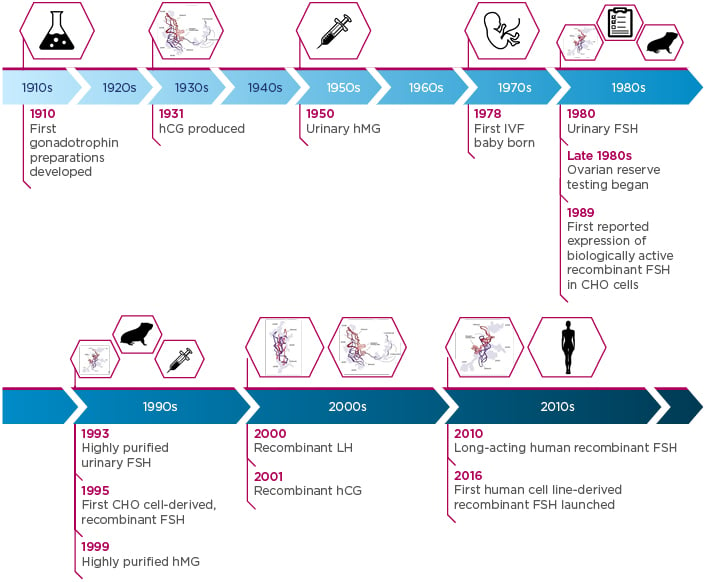
Figure 1: Timeline of gonadotrophin development from the start of the 20th century to the present day.1-6
CHO: Chinese hamster ovary; FSH: follicle-stimulating hormone; hCG: human chorionic gonadotropin; hMG: human menopausal gonadotrophin; IVF: in vitro fertilisation; LH: luteinising hormone.
FSH, hCG, and LH images adapted from Leão and Esteves.1
Glycosylation patterns play a key role in determining the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of gonadotrophin isoforms, impacting their receptor binding affinity, bioactivity, and clearance rate.8-10 The origin of the gonadotrophin (human-derived or Chinese hamster ovary [CHO] cell-derived) also has a significant impact on the glycosylation pattern of gonadotrophins. Human-derived gonadotrophins (such as urinary-derived products) and recombinant FSH (rFSH) derived from a human cell line have complex glycosylation patterns with a high sialic acid content; they contain a heterogeneous mixture of 2,3-linked and 2,6-linked sialic acid residues, whereas CHO cell-derived recombinant gonadotrophins have a less complex glycosylation pattern comprising 2,3-linked residues only,1,11 resulting in different PK and PD profiles in humans.12 With so many different gonadotrophins and protocols available to modern clinicians, the key question remains: ‘How exactly do you optimise OS treatment?’
Anti-Müllerian Hormone: The Backbone to Personalising Ovarian Stimulation
Doctor Elena Labarta
Infertility clinics across the world are faced with a heterogeneous population of women with varying characteristics, phenotypes, and genotypes, but all with the same goal: to have a healthy baby. Ovarian reserve can be measured to predict a woman’s response to OS, and treatment choice can be personalised based on this to maximise pregnancy success rates while minimising risks,2 costs, and patient burden. Considering this, it is of critical importance that the most reliable measurement of ovarian reserve is employed to accurately predict ovarian response, which will ultimately ensure that there is the highest probability of a successful pregnancy.2 Although a number of assessments have been proposed over recent years, AMH and antral follicle count (AFC) are widely accepted as the most reliable tools available for OS personalisation.2
A number of analyses have been carried out to compare AMH with AFC to decide the optimal biomarker of ovarian reserve. Broer et al.13,14 demonstrated that, in two individual patient data meta-analyses of observational trials, both AMH and AFC had similarly high performance in predicting poor and excessive ovarian response as single tests compared with age alone. When looking at AMH and AFC measurements between seven different centres, Anderson et al.15 found that AFC showed substantial variation, whereas AMH had minimal variation when measured in a central lab with an automated assay. Moreover, secondary analyses of two large, multicentre RCT (MERiT and MEGASET)16,17 have shown that AMH is superior to AFC for the prediction of ovarian response.18,19 MERiT and MEGASET compared rFSH and HP-hMG in gonadotrophin-releasing hormone (GnRH) agonist and antagonist cycles, respectively;16,17 post-hoc analyses of both trials showed that, compared with AFC, AMH correlated strongly with the number of oocytes retrieved in the majority of the centres included in these studies.19 In addition, the MEGASET trial showed that AMH had a higher capability for prediction of both poor and high response to OS and better performance than AFC, FSH, or inhibin B.18 Collectively, these results indicate that AMH is less variable and has a greater correlation with ovarian response than AFC,15,18,19 and it can be considered the single biomarker of choice for prediction of ovarian response.
Although the latter studies indicate that AMH correlates well with oocyte yield, some concerns have been raised regarding the variability of AMH levels measured as a direct result of the assay method and the stability of samples.20 Many different assays have been used previously to measure AMH levels; however, there has been a move from manual assays (ELISA) to automated assays (Elecsys® [Hoffmann-La Roche, Basel, Switzerland], Access [Beckman Coulter, Brea, California, USA], and VIDAS® [bioMérieux, Marcy l’Etoile, France]) in recent years.21-23 It should be noted that a lack of standardisation has been observed between the automated assays, with Access being found to systematically give higher values by an average of 10% compared with Elecsys.24 Despite this, the automated assays have provided a solution to the issue of analytical variability and more accurate AMH measurements can now be generated in a reduced amount of time.25 Automated assays also have a number of key advantages over manual assays; for instance, it is well known that with previous manual assays AMH was not stable under some storage or assay conditions. In contrast, Elecsys has proven to have no issues with sample instability for both sample collection type and storage conditions.21,22
AMH variability within individuals is another concern raised by clinicians.20 Variability is anticipated due to biological characteristics, reproductive factors, and environmental or lifestyle factors, and clinicians should take these into consideration when assessing a patient’s ovarian reserve test results.2 AMH levels are also known to fluctuate throughout the menstrual cycle; however, fewer fluctuations have been observed in larger trials as compared with smaller studies, and the variation is not considered large enough to be clinically relevant.25,26 It should also be noted that inter and intraindividual biological variability exists in other frequently used biomarkers, such as bilirubin, ferritin, urea, and high-density lipoprotein cholesterol,25,27-29 and the intraindividual variability seen with AMH26 is less than with these other biomarkers.
As well as predicting responses to OS, AMH and ovarian reserve tests can be used to personalise OS treatment;2 patient-tailored FSH dosing using AMH as a biomarker has been demonstrated with follitropin delta.30 To achieve this, it was necessary to first establish a dose–response relationship between exogenous FSH and ovarian response.31 A recent Phase II trial31 demonstrated a linear dose–response relationship with oocytes retrieved. Moreover, dose–response modelling indicated that AMH levels influence the predicted number of oocytes retrieved for various doses of follitropin delta (Figure 2).31 The model further demonstrated that AMH levels and body weight alone were sufficient biomarkers to personalise the follitropin delta dose,31 and this was then validated in a RCT.30 In summary, AMH is the key biomarker for predicting ovarian response to stimulation and, with this strategy, individualisation of ART stimulation protocols (including choice of regimens and dose adjustments) is now possible.
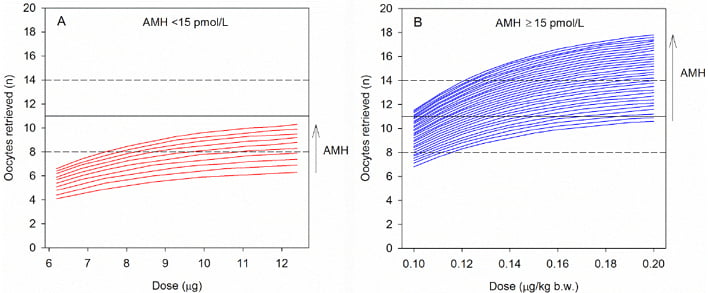
Figure 2: Dose–response model that shows the predicted number of oocytes received with different doses of follitropin delta and an anti-Müllerian hormone measurement of A) <15 pmol/L or B) ≥15 pmol/L.
The black horizontal dotted lines represent the target range of 8–14 oocytes retrieved.
AMH: anti-Müllerian hormone; b.w.: body weight.
Republished with permission of Nova Science Publishers Inc, from “Using AMH for determining a stratified gonadotropin dosing regimen for IVF/ICSI and optimising outcomes”, published in “Anti-Müllerian Hormone: Biology, Role in Ovarian Function and Clinical Significance”, Arce et al., publication date 2015, copyright 2004–2018; permission conveyed through Copyright Clearance Center, Inc.31
New Insights into Highly Purified Human Menopausal Gonadotrophin: MEGASET HR
Doctor Michael Alper
Different and individualised criteria inform personalisation of OS for each patient; based on each patient’s unique profile, treatment can be tailored by selecting the most appropriate gonadotrophin and deciding on the best administration dose. There is an increased emphasis in current clinical practice on selecting personalised treatment paradigms that are evidence-based and hence data-driven. Accordingly, the choice of gonadotrophin for each patient should also be evidence-based.
Patients with a high ovarian response (defined as patients who produce >15 oocytes in response to OS) experience unique problems due to the excessive production of oocytes and high oestrogen levels, which lead to an increased risk of ovarian hyperstimulation syndrome (OHSS), cycle cancellations, and a subsequent delay in time to pregnancy.32 Previous studies (EISG,33 MERiT,15 and MEGASET17) investigated HP-hMG versus CHO cell-derived rFSH treatments in IVF and intracytoplasmic sperm injection (ICSI), and further analysis of the data provided evidence to generate the hypothesis that HP-hMG may be efficacious, with an advantageous safety profile in high responders. Advancing this, Arce et al.34 carried out a retrospective analysis of the data collected in MERiT and MEGASET, investigating ovarian response and clinical outcome in potential high responders treated with either HP-hMG or rFSH. Results indicated that, compared with rFSH, HP-hMG was associated with a lower mean number of oocytes but a significantly lower incidence of high response (defined as >15 oocytes) and increased live birth rate per embryo transfer. The authors concluded that the specific gonadotrophin chosen for treatment has a direct effect on high response rate and, therefore, may influence clinical outcomes in high responders.34 Many fertility experts recommend the use of GnRH antagonist protocols in high responders and those at risk of OHSS; however, there are still limited data to support which gonadotrophin should be used.35-37
The MEGASET HR trial38 was set up to investigate specific gonadotrophin regimens in high responders. The study objective was to demonstrate non-inferiority of HP-hMG (Menopur® [Ferring Pharmaceuticals, West Drayton, UK]) versus rFSH (Gonal-f® [Merck Serono SpA, Modugno, Italy]) with respect to ongoing pregnancy rate in women undergoing OS following GnRH treatment. The study was a randomised, assessor-blind, non-inferiority clinical trial carried out at infertility centres across the USA. Patients predicted to be high responders (defined based on serum AMH levels) were enrolled to undergo IVF or ICSI treatment using a GnRH antagonist protocol with a fresh, single blastocyst transfer.38 The methods and results of the study will be reported in full at a later date and are consequently not included in this symposium review.
Follitropin Delta: Ovarian Stimulation with Efficacy and Safety at its Core
Doctor Jon Havelock
Ovarian response to stimulation is variable and unexpected extreme responses have both efficacy and safety implications.39 To minimise these risks, there is a need to predict ovarian response prior to OS.39 The success and safety of ART depends on a balance of obtaining enough oocytes for a sufficient number of embryos to transfer while avoiding too many oocytes in order to reduce the risk of OHSS.28 Several attempts have been made over recent years to predict ovarian response and tailor the starting dose of FSH using various biomarkers;40-45 however, many studies have used surrogate primary endpoints for ART outcomes and trial subject inclusion criteria have not been sufficiently robust to generalise results obtained to a broader patient population. To succeed, there is a need for a data-driven model validated in a large, prospective RCT.
Follitropin delta is a unique human rFSH that differs from the existing available FSH preparations. Although it has an identical amino acid sequence to urinary and CHO cell-derived FSH, follitropin delta is the first human cell line (PER.C6® [Crucell Holland BV, Leiden, Netherlands])-derived FSH with a complex, individual glycosylation pattern that closely resembles that of natural human FSH.46 The complex glycosylation of human-derived rFSH demonstrates a clearance rate and receptor binding profile that differs from other forms of rFSH.8-10 Investigational studies that looked at the PK and PD profile of follitropin delta have confirmed that, in comparison with the CHO cell-derived rFSH follitropin alpha, an equal international unit (IU) dose of follitropin delta (as determined by the Steelman–Pohley assay in rats) is not equally bioactive in humans.12 In fact, an equal IU dose of follitropin delta has a different PK and PD profile to that of follitropin alpha, resulting in a higher mean serum FSH concentration, higher oestradiol levels, and a higher median number of follicles than follitropin alpha.12 As a result, the Steelman–Pohley assay is not appropriate for measuring follitropin delta activity in humans and, therefore, follitropin delta is dosed in micrograms.12
Data from a Phase I trial47 were modelled and this revealed that the number of oocytes retrieved (when administering a constant follitropin delta dose) decreased with increasing body weight. Therefore, for the purpose of developing a validated dosing algorithm, it was most appropriate to calculate the dose of follitropin delta using body weight.47 Furthermore, the dose–response model evaluated multiple ovarian biomarkers and demonstrated that only AMH and body weight were necessary to maximally predict the ovarian response following follitropin delta treatment.31 Subsequently, Phase II studies were conducted to determine appropriate dosing for patients with either low AMH (<15 pmol/L) or high AMH (≥15 pmol/L), which led to the development of the follitropin delta dosing algoirithm.31 The rationale for the development of the algorithm was to affect the predefined optimal OS to maximise pregnancy rates, while minimising the risk of OHSS or extremes of ovarian response.30
The ESTHER30,48 programme, which consisted of two Phase III trials, has been carried out to support the efficacy and safety profile of follitropin delta and to prospectively validate the dosing algorithm for OS.30 ESTHER-1 was the first study and was a randomised, multicentre, assessor-blinded, controlled, non-inferiority trial comparing the treatment strategy of individualised follitropin delta dosing with that of conventional follitropin alpha dosing for IVF/ICSI. The study used a GnRH antagonist protocol with a single blastocyst transfer, and the key inclusion criteria were women aged between 18 and 40 years with a BMI of 17.5–32.0 kg/m2 and regular menstrual cycles of 24–35 days. The women had to be undertaking their first ART cycle and diagnosed with either tubal infertility, unexplained infertility, or endometriosis Stage I/II, or had to have partners diagnosed with male factor infertility. There were no AMH level restrictions but early follicular phase serum levels of FSH were required to be ≤15 IU/L. Ovulatory patients with polycystic ovaries were also included in the study. The coprimary endpoints of the study were ongoing pregnancy rate (10–11 weeks after transfer) and ongoing implantation rate (a predefined non-inferiority margin of -8.0%), while the secondary endpoints included distribution of ovarian response, proportion of patients with extreme responses (hypo and hyper-responses), live birth rate, early and late OHSS, and early OHSS and/or preventive interventions.30 ESTHER-2,49 a continuation of ESTHER-1, was a safety immunogenicity study, allowing for up to two further OS cycles in women who did not achieve an ongoing pregnancy in ESTHER-1. In terms of the dosing of follitropin delta, this was calculated using an algorithm: in women with AMH ≥15 pmol/L, the daily dose was calculated according to the actual AMH value and body weight, while in women with AMH <15 pmol/L, a fixed daily dose of 12 μg was administered irrespective of body weight. The dosing algorithm sets the maximum daily dose at 12 μg in the first treatment cycle.30 Once calculated according to AMH levels and body weight, the daily dose of follitropin delta was fixed throughout stimulation and was only adjusted in subsequent cycles of OS according to the response seen in the previous treatment cycle.50
The main efficacy results of ESTHER-1 were presented during the symposium (Figure 3): the study met its coprimary endpoints of non-inferiority, with similar data shown between follitropin delta and follitropin alpha for both ongoing pregnancy and ongoing implantation.30 There were also similar results for the secondary endpoints of live birth rate and oocyte yield between the two gonadotrophins, though the number of oocytes obtained was more homogeneously distributed in relation to AMH levels in the follitropin delta group. In terms of OHSS and OHSS preventive interventions, with increasing levels of AMH, the risk of OHSS and/or requiring preventive intervention increased differently in the two treatment arms. These results provided additional evidence to support the use of follitropin delta with the individualised dosing algorithm.30 Cumulative OHSS data and long-term neonatal outcomes data from ESTHER-1 and ESTHER-2 were also presented; however, these will be published in full at a later date and are consequently not included in this review. Overall, the data from the ESTHER programme demonstrate a favourable benefit–risk profile with follitropin delta treatment, especially in women with high AMH.51 As a result of the ESTHER-1 trial, follitropin delta and its dosing algorithm have now been validated in a RCT and, to date, this is the only gonadotrophin with an approved ovarian reserve biomarker-based algorithm for dosing.30
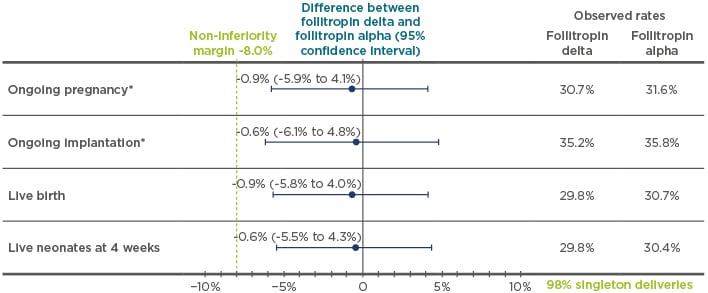
Figure 3: The outcomes of the pregnancy endpoints (ongoing pregnancy, ongoing implantation, live birth, and live neonates at 4 weeks) of ESTHER-1.
*Trial powered to at least 80% to establish non-inferiority; non-inferority limit prespecificed at -8.0% for both coprimary endpoints.
Adapted from Andersen et al.30
It has been demonstrated that not all rFSH are the same and follitropin delta is different due to its unique PK and PD profiles. The ESTHER programme has successfully validated the safety and efficacy profile of follitropin delta. When used in conjunction with the individualised dosing algorithm based on AMH and body weight to establish a predictable ovarian response and reduce the risk of OHSS, follitropin delta provides the same pregnancy outcomes but with an improved safety profile.
Conclusion
Professor Bart Fauser
The symposium concluded with a review by the chairperson, Prof Bart Fauser, of the key points discussed. AMH is the biomarker of choice for predicting ovarian response for individualising the dose of gonadotrophins. The AMH level can also influence the choice of gonadotrophin in different patient types; this has been investigated in the MEGASET HR trial with the established gonadotrophin HP-hMG. The value of AMH in predicting OS and enabling personalised treatment can be seen in its use in the follitropin delta dosing algorithm, which uses a data-driven algorithm based on AMH and body weight and has resulted in a favourable benefit–risk profile, especially in women with high AMH levels.
As personalised treatment approaches are becoming the norm in medicine, including in infertility treatment, Ferring continues its scientific commitment to innovation in ART to support clinicians treating patients with infertility.








