Meeting Summary
This symposium took place during the 2018 Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE). It centred on the role of progestogens in the treatment of recurrent pregnancy loss (RPL) and in luteal support during assisted reproductive technology (ART), with consideration also given to the relevance of maternal adaptation in human pregnancy being under the control of progesterone and progestins. Focussing on the potential role of dydrogesterone (DYD) in the treatment of RPL, the speakers discussed the role of progestogens and how they might fit into the ESHRE guidelines for recurrent miscarriage, as the effect of this treatment approach continues to be debated. In particular, the presenters discussed whether DYD could address the current issues associated with this class of drugs; they presented evidence from the recent LOTUS I study comparing DYD with micronised vaginal progesterone (MVP) and whether the effects may be linked to supporting maternal immune adaptation for successful blastocyst implantation and the progression of pregnancy, the latter being assessed by the amount of CD4+ T regulatory cells in peripheral blood and the levels of local immune cell subsets and immunosuppressive molecules evaluated in endometrial biopsies. There remains a need for further trials to evaluate the benefits of administering progestogens from the luteal phase of pregnancy.
Do Progestogens Fit in the European Society of Human Reproduction and Embryology Guidelines for Treatment of Recurrent Miscarriage?
Professor Howard Carp
In the current ESHRE treatment guidelines,1 RPL is considered a homogeneous, single entity and is not classified according to prognosis or the number of losses. There is no personalised approach to classification or treatment.1 However, opinion remains divided. RPL is defined as the loss of at least two pregnancies; furthermore up to 50% of RPL cases do not have a clearly defined aetiology.2 Additionally, no information is provided in the guidelines about RPL patients who are resistant to treatment.1 There is insufficient evidence to date on the benefits of progesterone, human chorionic gonadotropin (hCG), or metformin as pharmacological interventions. Similarly, there is little evidence to support the clinical benefits of hysteroscopic myomectomy, adhesiolysis, polypectomy, and intramural myomectomy. In cases of hereditary thrombophilia, antithrombotics should not be used unless indicated for venous thromboembolism. Because of insufficient benefit–risk evidence, sperm selection, lymphocyte immunisation therapy, intralipid granulocyte colony-stimulating factor or intravenous Ig, steroids, anticoagulants (heparin or low-dose aspirin), and endometrial scratching are not recommended.
With regard to progestogen treatment, there are several questions: ‘Why should it work?’, ‘Why does it often fail?’, and ‘Does it actually work in clinical practice?’ The basis for their predicted success is that progestogens have both endocrine and immunomodulatory functions. Endocrine effects include endometrial decidualisation, improved implantation, inhibition of arachidonic acid release leading to reduced prostaglandin synthesis, reduced cervical stromal degradation, altered barrier function to cervical ascending inflammation or infection, reduced gap junction formation, and decreased expression of oxytocin receptors.3-5 The evidence for the role of progesterone deficiency in miscarriage is strong. A very early study by Csapo et al.6 in 1973 showed that luteectomy before 7 weeks causes spontaneous abortion. Mifepristone blocks the progesterone receptor, causing fetal death and placental separation. Furthermore, a defective corpus luteum may produce levels of progesterone that are too low to support endometrial ripening, implantation, or placentation.
There are two main reasons why progesterone treatment may be unsuccessful. First, structural malformations in the embryo can be a confounding factor. Around 70% of miscarriages show blighted ova, and it is not possible to tell whether the rudimentary embryo may have been structurally abnormal. A study by Philipp et al.7 using embryoscopy found developmental defects in 200 of 233 (85%) missed abortions, including anencephaly, encephalocele, spina bifida, syndactyly, pseudosyndactyly, polydactyly, and cleft hand and cleft lip. In this study, 56 out of 221 (25%) of karyotyped embryos had a normal karyotype. However, embryoscopy is an advanced technique that is not usually available; the more widely used technique, ultrasound, does not detect most anomalies (example shown in Figure 1). Another confounding factor is embryonic aneuploidy. Research has shown that 60% of sporadic miscarriages8,9 and 45% of recurrent miscarriages10 are due to chromosomal aberrations, including trisomies 16, 18, and 21; Turner syndrome (XO); and triploidy. In 2010, Rajcan-Saparovic et al.11 showed copy-number variation in 26 miscarriages with normal karyotype when comprehensive chromosomal analysis was used. The incidence of embryonic aneuploidy increases with maternal age.12
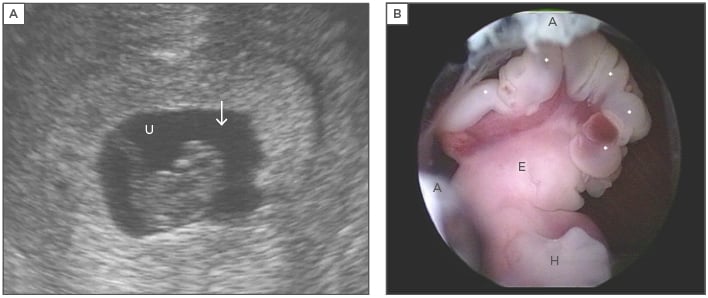
Figure 1: Structural malformations in the embryo that can lead to pregnancy loss.
Figure 1A: Endovaginal sonography prior to embryoscopy. The embryo of 17 mm, crown-rump length, showed no heartbeat. No abnormalities were identified sonographically. The arrow marks the head of the embryo. U: umbilical cord; Figure 1B: Embryoscopic lateral view of the upper portion revealed a well-preserved embryo with anencephaly. The exposed brain tissue (*) is still intact (exencephaly). The digital rays of the hand (H) are notched. Parts of the external ear (E) are clearly discernible. Remnants of the amnion are labelled (A). A normal karyotype was diagnosed cytogenetically (46,XX).
Adapted from Philipp T.18
Whether or not progesterone support can reduce RPL has been investigated in several studies, the majority of which showed positive results for progesterone, confirmed by a subsequent meta-analysis.13 In the recent randomised, double-blind, placebo-controlled PROMISE trial of progesterone in women with RPL (n=404 active treatment, n=432 placebo), there was a nonsignificant increase in live births in the active treatment group (65.8% versus 63.3%; risk ratio: 1.04; 95% confidence interval: 0.94–1.15).14 MVP has no beneficial effect in women with unexplained RPL; there is some evidence that DYD may be effective if initiated when fetal heart action is confirmed.1,15 However, as progesterone is important during implantation, DYD supplementation may be of benefit if it is administered from the luteal phase, rather than after a positive βhCG test. More trials are required to evaluate DYD and, specifically, its administration from the luteal phase onwards.
A recent randomised controlled trial of DYD (administered until Week 20 after viability was confirmed by ultrasound) showed a benefit in reducing the subsequent risk of miscarriage compared with placebo (risk ratio: 2.4; 95% confidence interval: 1.3–5.9),15 which was supported by a subsequent meta-analysis that found DYD was favoured compared with standard treatment.16 However, there is insufficient clinical evidence for the benefits of progesterone or hCG to reduce RPL.
Using an evidence-based approach, ESHRE guidelines recommend that progestogens should not be given if there is no evidence of effect.1 As the medical community does not know all of the confounding factors during clinical trials, data should be evaluated using an intent-to-treat basis, relying on randomisation to neutralise potentially confounding effects. However, using a personalised-medicine approach, physicians can rule out confounding factors to reach an accurate diagnosis and determine which patients may respond (e.g., assessing karyotype of abortus or previous miscarriages and allowing the patient to decide future treatment based on this information). Key points to consider after treatment failure include karyotyping of abortus or embryoscopy if the patient was in the first trimester, noting that intrauterine embryoscopy is a specialised technique. If the embryo was abnormal, the treatment should be repeated (preferably based on karyotyping of the last miscarried embryo). Paraffin-block analysis may also be required. In the event of repeat aneuploidy, preimplantation genetic testing for aneuploidy should be employed, followed by luteal support with DYD.17
In conclusion, the overall findings are that progestogens may prevent miscarriage of a viable embryo, while DYD may have additional advantages over progesterone in terms of efficacy. Study results may be confounded by fetal factors, but progesterone and DYD appear to support pregnancy via both anti-inflammatory cytokine and endocrine effects. Guidelines should be used to tailor treatment to the individual patient and if the patient miscarries despite treatment, it is advisable to audit the possible causes of treatment failure.
Luteal Support in Assisted Reproductive Technology: Could Dydrogesterone Become the New Gold Standard?
Professor Christophe Blockeel
Iatrogenic luteal phase defect is caused by supraphysiological steroid levels in stimulated cycles. Following a systematic literature review19 and a global survey,20 with regard to luteal phase support (LS) it seems that there are currently more questions than answers: ‘When to start?’, ‘When to stop?’, ‘What is the optimal duration?’, ‘How much support should be given?’, ‘What kind of support should be used?’, and ‘What is the optimal route of administration?’
DYD is a retroprogesterone, a stereoisomer of progesterone, with an additional double bond between carbons 6 and 7 (Figure 2).21,22 Differences in the structure of DYD and progesterone influence the potency and potential side effect profile of these progestogens. DYD has been used globally since the 1960s for several conditions related to progesterone insufficiency.23 It is estimated that the cumulative exposure for all indications from 1960–2017 is >113 million patients.17 Of these, it is estimated that >20 million pregnancies were exposed to DYD in utero.17 Overall, clinical and post marketing experience support the well-established and favourable benefit–risk profile of DYD in the approved indications,23 which include progesterone deficiencies (dysmenorrhoea, endometriosis, secondary amenorrhoea, irregular cycles, dysfunctional uterine bleeding, premenstrual syndrome, threatened miscarriage, habitual miscarriage, infertility due to luteal insufficiency, and LS as part of an ART treatment) and hormone replacement therapy.24
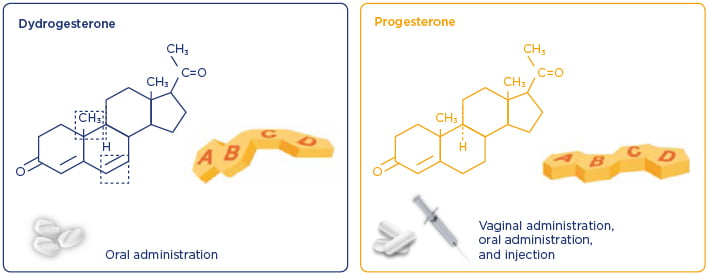
Figure 2: Differences in the structure of dydrogesterone and progesterone influence the potency and potential side effect profiles of these progestogens.21,22
Leading up to 2012, three prospective, randomised, controlled trials concluded that DYD was equally as effective as, or more effective than, MVP for LS in in vitro fertilisation (IVF).25-27 Thus, the 30 mg daily dose of DYD for the subsequent LOTUS I study17 was selected on recommendations of IVF specialists and based on the previous studies.
LOTUS I was a randomised, double-blind, double-dummy, multicentre, multinational study comparing the efficacy, safety, and tolerability of oral DYD 30 mg daily versus MVP capsules (600 mg daily) for LS in IVF.17 The primary objective was the improvement of pregnancy rate (confirmed by the presence of fetal heartbeat at 12 weeks’ gestation, determined by transvaginal ultrasound). Secondary objectives included a positive pregnancy test on treatment Day 15 after embryo transfer, incidence of live births, newborn assessments (sex; Appearance, Pulse, Grimace, Activity and Respiration [APGAR] score; weight; height; head circumference; abnormal findings of physical examination; and any malformations), safety, and tolerability. Demographic and baseline characteristics were similar between treatment groups. In the total treatment population (DYD n=497; MVP n=477 [full analysis set]), DYD was shown to be non-inferior to MVP regarding the presence of fetal heartbeat at 12 weeks of gestation. There was a nonsignificant, numerical difference in favour of DYD regarding live birth rates.
In conclusion, the LOTUS I trial showed that oral DYD was non-inferior to MVP for the presence of fetal heartbeat at 12 weeks of gestation, and that rates of positive pregnancy test, clinical pregnancy, live births, and newborn assessments were similar between the two treatment groups.17 Given that oral DYD treatment had a similar safety profile to MVP in LOTUS I, with no new safety concerns identified during the study, oral DYD may replace MVP as the standard of care for LS in IVF because of the ease of oral administration.
Immunomodulation in Early Pregnancy
Professor Petra Arck
Fetal programming is an emerging concept that links environmental conditions during embryonic and fetal development with risk of diseases later in life. Mammalian pregnancy is a unique situation. The specific placental human leukocyte antigen (HLA) expression repertoire can trigger a maternal immune response, which renders the fetus susceptible to rejection. This is associated with HLA expression on trophoblast cells: a combination of negative or low expression of class Ia antigens (HLA-A, HLA-B, HLA-C), expression of class Ib antigens (HLA-E, HLA-F, HLA-G), and lack of class II antigens.28-31 Pregnancy success results from complex adaptations, including upregulation of immunosuppressive molecules in decidual stroma cells, reduced galectin-1 expression in spontaneous abortion, the unique appearance of tolerogenic dendritic cells in the decidua of early human pregnancies, and the generation of CD4+ regulatory T cells locally and in the blood.31-35
The concept of Th1 (proinflammatory) and Th2 (anti-inflammatory) balance has long been thought to be important for understanding successful and failed pregnancies, but a new paradigm is emerging.36 Balances in favour of Th1 responses can lead to epidural-associated fever, pre-eclampsia, RPL, preterm labour, and gestational diabetes.37,38 Maternal adaptation to pregnancy is modulated by progesterone and other progestins. For example, systemic DYD administration upregulates decidual galectin-1 expression in mice, progesterone robustly increases the frequencies of CD4+ T regulatory cells in mice, progesterone and DYD support the tolerogenic phenotype of dendritic cells and trigger the release of immunosuppressive molecules in mice, and decreased progesterone levels are associated with increased fetal loss and low levels of both galectin-1 and CD4+ T regulatory cells.39-42 Using high-parameter functional profiling by mass cytometry, combined with a single-cell signalling-based Elastic Net algorithm, it has been shown that there are dynamic changes in the peripheral immune system during the course of pregnancy.43
Immune adaptations are finely tuned to an ‘immune clock’ that regulates immune cell function to maintain pregnancy.43 Analysis of these interrelated, chronological immune events has revealed the critical role of the IL-2-dependent STAT5ab signalling pathway in modulating T cell function during term pregnancies.43 This has also led to an understanding of how deviations from normal immunoregulation can lead to adverse outcomes in pregnancy. Thus, the future of obstetric care to reduce RPL would involve a multidisciplinary team, including not just paediatricians, nurses, and obstetricians but also immunologists, reproductive immunologists, reproductive endocrinologists, laboratory medicine specialists, gene therapy specialists, and biostatisticians.
In summary, maternal adaptation to pregnancy is under the control of progesterone and progestins. Systemic progestogen supplementation may significantly support the maternal immune adaptation for successful blastocyst implantation and pregnancy progression. Candidates to evaluate the systemic effect of progestogens on maternal immune adaptation in human trials should include monitoring of both CD4+ T regulatory cells in peripheral blood and local immune cell subsets and immunosuppressive molecules in endometrial biopsies.
Conclusion
To conclude, the management of RPL is evolving. Current guidelines do not use a personalised approach to treatment, as RPL is classified as a single entity with no consideration of the multitude of underlying causes. There is evidence for the beneficial effects of progestogens in RPL, in particular at an early stage of pregnancy (LS), with DYD showing advantages over standard MVP, in part because of the ease of oral administration. There is also a strong immunological rationale for systemic progesterone supplementation to increase blastocyst implantation and the progression of pregnancy, demonstrated by monitoring CD4+ T regulatory cells, local immune subsets, and immunosuppressive molecules. Further studies to investigate the evolving treatment of RPL are warranted.








