Abstract
Umbilical cord haematoma (UCH) and uterine torsion are extremely rare complications in pregnancy. These abnormalities may present in acute and spontaneous conditions, however, they should not be neglected in clinical practice when monitoring an abnormal fetal heart without other suspects. The authors hereby report a rare case of UCH and uterine torsion as well as a review of the literature. A female, aged 35 years old (gravida 1, para 0), was admitted to the Emergency Department of Tu Du Hospital, Ho Chi Minh City, Vietnam, due to term gestation without complaints. They had an uncomplicated pregnancy, except a large uterine fibroid and cervical pessary which prevented pre-term birth from 28 weeks of gestational age. A very rare complication of UCH was revealed accidentally following emergency caesarean section associated with abnormal fetal heart rate tracing. Asymptomatic uterine torsion was noticed at the same time as this dramatic event. Pre-operative diagnosis of two rare complications was missed; hence, the authors timely delivered the baby based on another modality of management, computerised cardiotocography. In conclusion, UCH along with uterine torsion is difficult to diagnosis due to its rarity; it is usually an incidental finding. Moreover, no available imaging modality could investigate UCH prior to delivery. Surveillance on fetal heart rate monitoring may be helpful in this fatal situation.
Key Points
1. Uterine torsion can occur in pregnancy with large fibroid, which can lead to an umbilical cord haematoma.
2. Umbilical cord haematoma may be carefully considered in cases of neonatal acidosis at term if other aetiologies are not found, since this pathology suddenly causes abnormal fetal heart rate.
3. Strict surveillance on cardiotocography is necessary for all suspected cases, and caesarean section is needed in an emergency due to the high rate of fetal mortality.
INTRODUCTION
Abnormalities of the umbilical cord are very common in pregnancy. These include marginal insertion, velamentous cord insertion, umbilical cord true knot, umbilical torsion, and many more.1-3 Nevertheless, term umbilical cord haematoma (UCH) is an extremely rare complication; it is almost unheard of. An average obstetrician may face it once in their lifetime. The rate of this accident occurs in around 1/5,500–1/11,000 births.4-6
To the authors’ knowledge, the first case was reported in 1981 by Benirschke K et al.7 At the time of writing this article, the authors found about 10 cases published in the last 10 years from around the world (Table 1).
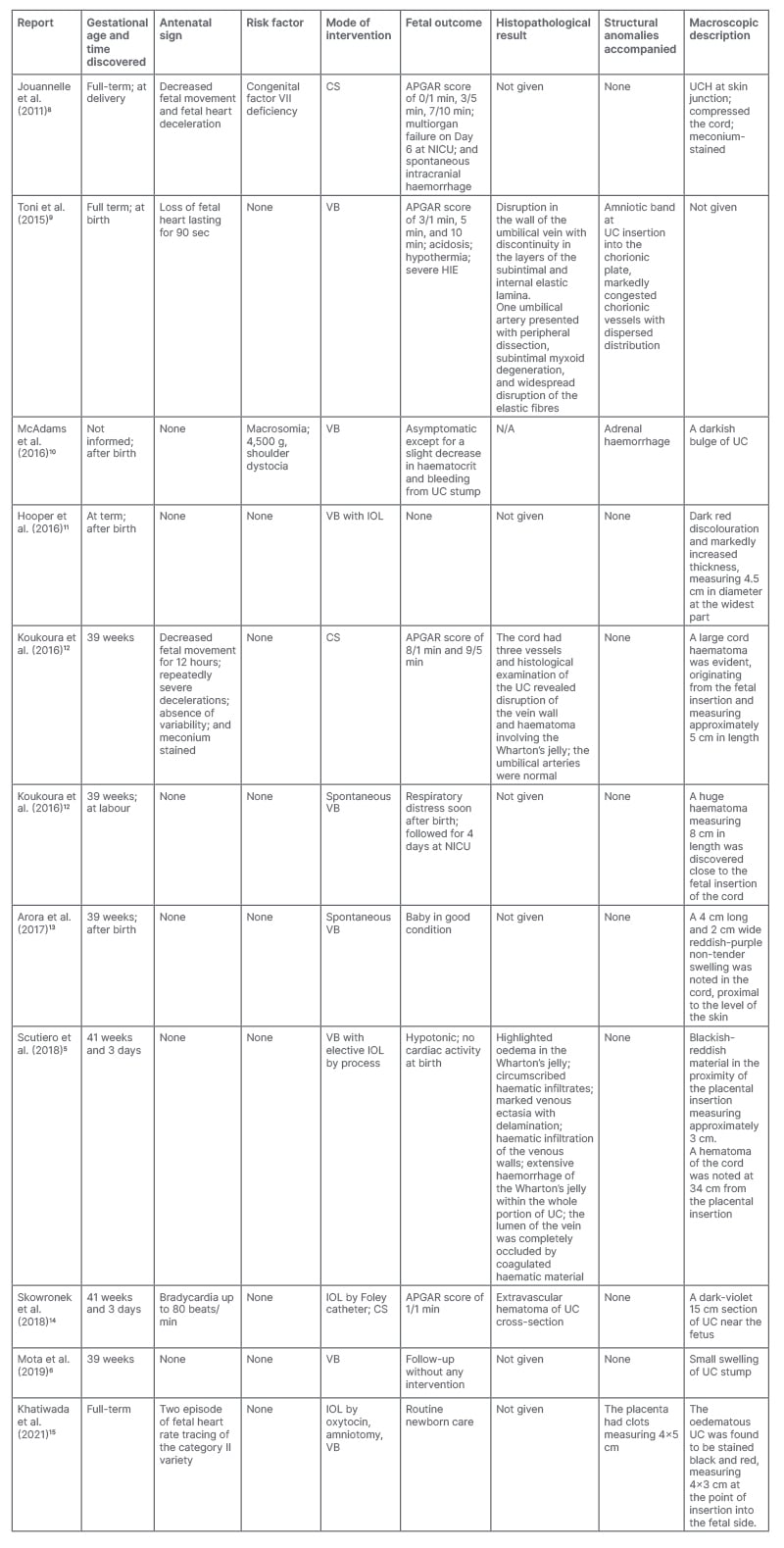
Table 1: Review of the literature of umbilical cord haematomas that have been reported in last decade.
APGAR: Appearance, Pulse, Grimace, Activity, and Respiration score; CS: Caesarean section; HIE: hypoxic ischaemic encephalopathy; IOL: induction of labour; NICU: neonatal intensive care unit; UC: umbilical cord; UCH: umbilical cord haematoma; VB: vaginal birth.
The umbilical cord is the vital line between placenta and fetus; it is the attachment of fetoplacental circulation. The structure of a normal umbilical cord includes two arteries and one vein. It is surrounded by Wharton’s jelly, which is 40–60 cm in length and the mean cord thickness is 1.21 (±0.39) cm.16,17 At term, maternal blood flow through the placenta is nearly 600–700 mL/min.18 Certainly, if there is an interruption in the maternal blood supply, a lethal event of the fetus in utero is unpreventable. UCH is defined as the incomplete extravasation of blood due to an unpreventable rupture of the umbilical vasculature, which mechanically compresses the vessels, resulting in the interruption of the blood supply to the fetus and leading to severe acute fetal hypoxia. The elastic fibres and reduction in stromal myfibroblasts are abnormally shaped and blunted instead of stellate.9
Regardless of pathogenesis, UCH is sequelae of the accumulation of blood into the Wharton’s jelly due to partial laceration of the umbilical vein. In almost all cases, it occurs spontaneously and without clear aetiology, or it may be secondary from trauma during labour. A certain diagnosis is usually determined at delivery. Meanwhile, other risk factors include anatomic abnormalities, invasive fetal procedures such as amniocentesis, oligohydramnios, shortness of structure, traction of cord, post-maturity, chorioamnionitis, long-lasting fetal infection, or in association with disorder of coagulation factors. Degeneration of the umbilical cord caused by a meconium-stained liquor or funisitis may worsen the factors described.2,4,8,9,19
Generally, diagnosis of UCH is very difficult in clinical practice because of its rarity. Almost all cases were determined postnatally. Moreover, it is usually missed on sonography unless it is accompanied by an abnormal Doppler flow, or other structural anomalies such as an umbilical cord cyst, haemagioma, or a cord that is swollen more than the normal size.5,20,21
Umbilical abnormalities are not strictly related to life-threatening gestational accidental deaths. In late first and second trimesters, these abnormalities can cause sudden and unexplained fetal death. Later, this complication is responsible for severe fetal distress and causes approximately 50% of perinatal deaths in this situation. Evolution is associated with hypoxic encephalopathy, which increases mortality rate if a diagnosis is missed. Therefore, careful examination of placenta and cord is considered in such cases.3,9,22
PRESENTATION
A 35-year-old female who was primigravida was admitted to a tertiary hospital due to term gestation at 39 weeks and 2 days without complaints. Their medical history recorded hyperthyroidism well-controlled by medical treatment. They had a large fibroid, which was accidentally discovered before pregnancy. They got pregnant after 7 years of primary infertility. The results of their Group B streptococcal test was positive. The patient underwent cervical support by pessary from Week 28 for the prevention of preterm risk factors. They had already received two 12 mg doses of betamethasone for fetal lung maturity. Prior to admission, the patient had also not received cordocentesis during antenatal care.
At hospitalisation, the patient had normal vital signs, without remarkable signs of labour. Digital examination showed that an Arabin® pessary (Dr. Arabin, Witten, Germany) was in place without cervical dilation, and no uterine contraction appeared on cardiotocography (CTG). Routine laboratory tests were within in normal limits.
An ultrasound examination showed a vital fetus with appropriate development for gestational age and a normal Doppler flow in the middle cerebral artery, as well as in the umbilical artery. Furthermore, the structure of the placenta, umbilical cord, and fetal morphology were also normal. The fibroid was approximately 8×10 cm in size and located at the posterior, lower segment of the uterus. However, during routine management, the fetal heart rate monitoring (FHRm) on CTG and computerised CTG was abnormal, showing recurrent variable decelerations accompanied by minimal baseline variability (Figure 1A and 1B). Consequently, CTG was classified as Group 2, following classification from the American College of Obstetricians and Gynecologists (ACOG). The lowest short-term variability (STV) was 1.5 msec (the normal value on the monitor was >3.5 msec [ Figure 1C ]). The fetus’s health was not good; although, the fetus was reanimated by changing the mother’s lying position to the left lateral side. Intravenous infusion with lactated Ringer’s solution and supplemental oxygen was given to the mother at 6 L/min during 30 min. Therefore, the authors immediately decided to perform an emergency Caesarean section (C-section) to deliver the baby, which the patient allowed.
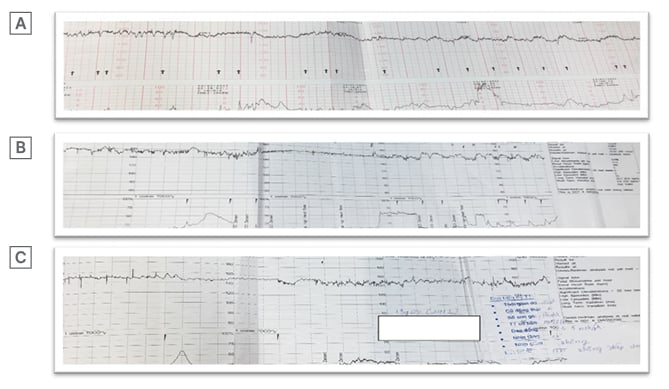
Figure 1: Image showing cardiotocography and computerised cardiotocography at admission (A), surveillance (B), and prior to Caesarean section (C), respectively. All were classified followed Group 2 by the 2009 American College of Obstetricians and Gynecologists (ACOG) classification due to low variability.
Interestingly, at laparotomy, the uterus was rotated at 90 ° right axis. After an incision in the lower segmental uterus, the authors promptly saved a healthy male infant, who weighed 3,200 g. The Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) score of the baby was 7 and 8 at 1 and 5 minutes, respectively. At the same time, the authors noticed that the umbilical cord was reddish, even though amniotic fluid was a normal colour. No mass was found on the umbilical cord, but a haemorrhage was discovered that followed a ‘flat’ form along the length of the placental cord insertion to fetal cord insertion (Figure 2A). The fetal surface placenta was also haemorrhagic in macroscopic appearance.
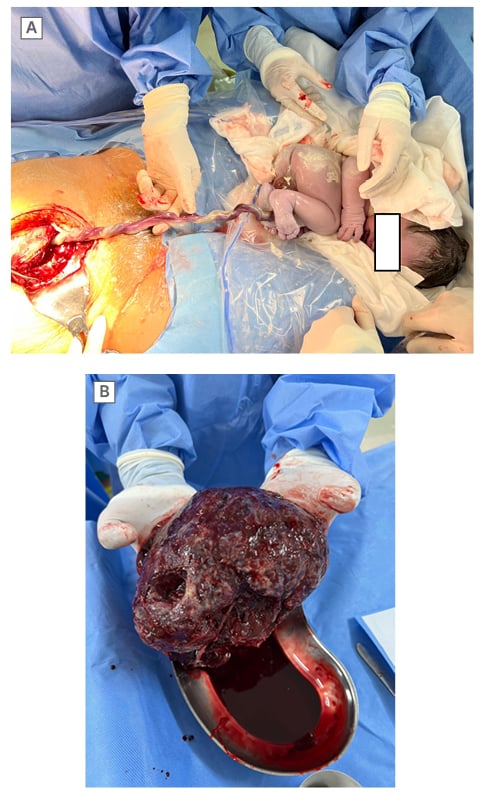
Figure 2: Macroscopic images of umbilical cord and placenta at Caesarean section.
A) The UCH discovered at the time of the C-section. B) The placenta without detachment at maternal surface.
C-section: Caesarean section; UCH: umbilical cord haematoma.
After de-torsing the uterus, haemostasis of the bleeding surface at the placental site was performed. In addition, the authors performed a ligation of bilateral uterine arteries to prevent late postpartum haemorrhage due to uterine torsion. Due to a large intramuscular fibroid located at posterior lower uterine segment, the authors promptly decided not to carry out a myomectomy in this situation. Following all these procedures, the uterus contracted effectively.
Post-operatively, the baby’s umbilical cord was normally dry in 48 hours and there was no additional haematoma. Due to neonatal jaundice, blood samples for serum bilirubin were taken from the baby, as well as screening the congenital diseases as a matter of routine. Results were within normal limits.
Both mother and baby were discharged home on Day 5 post-partum. The patient was scheduled for a routine 1 month follow-up and their health was still stable.
DISCUSSION
In the presented case, UCH was accidentally found during the C-section and could have been caused by uterine torsion. The haemorrhage appeared in placenta and umbilical cord; however, the authors did not find any haematoma in the placenta.
However, the patient had no symptoms for clinical diagnosis of uterine torsion prior to C-section. This is probably because the malposition was in an acute condition, so vascular damage did not have enough time to develop. Moreover, the uterus was not necrotic because of lack of blood supply. Necrosis is commonly mentioned as a complication of gradual uterine torsion, not only during pregnancy, but also in patients who are pregnant. Additionally, urgent complications such as haemorrhages, coagulopathy disorders, and sepsis are also described.23
In fact, uterine torsion, which leads to mortality for both mother and fetus, is extremely rare. Some articles that discuss this condition show that it is diagnosed in the second or third trimesters, or even at term gestation, similar to the case presented by the authors.23 This fatal condition typically has acute symptoms such as vomiting, vaginal bleeding, excruciating abdominal pain, and even uterine necrosis due to irreducible torsion. It rarely presents without symptoms or signs.24-26
Rotation ranges between 45 ° and 72 ° compared with its longitudinal axis.27 Interestingly, rotation along with horizontal axis or transversal axis has also been reported. Risk factors of malposition include morphologic anomalies, pelvic tumours, pelvic adhesion, asymmetry in the transverse diameter of the uterus because of transverse presentation, lateral fibroids, bicornuate uterus, and multiple pregnancies.26 In the authors’ case, the patient had no abnormality of anatomic structure, which caused axial torsion, and they found an enlarged fibroid of approximately 8×10 cm. Some reports revealed that a huge fibroma could be attributed to uterine torsion because of asymmetric weight distribution, which is commonly found in non-gravid uteruses and in postmenopausal periods due to atrophy of uterus. Diagnosis by imaging technology such as MRI can be helpful in this complication.23,28,29 At the time of writing, the authors were not able to find any case reports related to uterine torsion in combination with a cervical pessary. Therefore, they could not conclude that a cervical pessary is a risk factor for uterine fibroid in this case. The authors are waiting for further investigations about this mechanism.
Similar to all cases reported, no clinical sign or image scan contributed to detect antepartum UCH in the author’s case. However, reduced fetal movements were reported in most cases.5 With this patient, the authors accidently discovered this intraoperative complication due to other indications. To be precise, the authors performed a C-section following abnormal FHRm (Figure 1A–C). Accordingly, STV was in association with hypoxia and increased acidaemia in the umbilical cord blood sampling, which has been described by several authors.30,31 The cut-off point of STV is still unclear, but some reports have agreed that a low STV means that there is a poor prognosis for the fetus and needs further investigation.31
Fortunately, in the authors’ case, they were promptly informed of this due to FHRm, and thus, they could intervene rapidly and save the baby’s life. In this case, UCH was discovered that followed a flat feature form and the mass was not palpated on umbilical cord. According to literature, UCH located in the proximal portion of the fetal cord insertion causes severe obstruction of blood flow (Table 1). In 2009, Towers et al.32 and Barbati et al.33 also reported similar cases, which were discovered spontaneously by a fetal heart monitor in the USA and Italy. Recently, Koukoura et al.12 and Khatiwada et al.15 also reported on two cases that were similar to this event, with the precious experience on continuous FHRm in surveillance of labour.
Similar to some cases reported in literature, uterine torsion in relation to large fibroids was found at upon laparotomy in the full-term pregnancy.34 Spontaneous UCH was diagnosed following a C-section as in the author’s case. Unfortunately, they did not have histopathological results compared with other reports as well as legal evidence in situation of fetal death.9,14
The newborn was in good condition after birth and the neonatal course was uneventful. No anomalies were observed on examination. Both the mother and baby were in a healthy condition after a one-month follow-up.
CONCLUSION
UCH is an extremely rare entity in obstetric practice. Particularly UCH, in the context of uterine torsion, is an unheard phenomenon in the literature. In reality, antenatal diagnosis of two rare complications is not commonly recognised. Furthermore, fetal prognosis is poor if urgent extraction of fetus is delayed. At the time of writing, none of tool can approach in determination of this consequence. However, obstetricians should take extra care in evaluating pregnancies with large uterine fibroids, as well the accident of UCH to rule out cases of unexplained hypoxia. Following fetal heart monitoring managed by computerised CTG was strictly useful for a timely interpretation. Further data is required to elucidate this tissue.







