Abstract
Endometriosis is a chronic condition that affects ˜10% of young women worldwide. Pain and infertility are the two most common features of the disease. The condition appears to be sex hormone-dependent, although a subset of females with the condition still experience symptoms post-menopause. The aetiology of endometriosis induction still remains elusive, and surgery to remove the lesions often fails to cure the condition, as the lesions often reappear. The lesions contain stromal cells, blood vessels, nerves, and numerous mast cells. In some respects, endometrial lesions resemble a chronic fibrotic scar-like tissue that does not resolve. Studies in other fibrotic abnormal healing conditions have revealed that targeting mast cells, as a central component of what is called a ‘neural–mast cell–fibroblast’ axis, by repurposing asthma drugs can prevent induction of the abnormal healing phenotype. Given the similarities between conditions with abnormal healing phenotypes and endometrial lesions, it is postulated that taking a similar approach to target endometrial lesion mast cells could exert a benefit for patients with endometriosis. This review also outlines approaches to assess the likelihood that targeting mast cells could lead to clinical trials using such ‘repurposed’ mast cell targeted drugs.
PREAMBLE
This review is intended to focus on the rationale and feasibility of targeting mast cells in endometriosis, and discussing selected findings in the literature related to this focus. Due to space limitations, it is not intended to be an exhaustive review of all aspects of endometriosis. Thus, the reader is encouraged to read the large number of recent reviews in the literature which address other aspects of this complex disease to complement the discussion below.
THE CLINICAL PROBLEM
Endometriosis is an inflammatory condition that affects ~10% of young women of reproductive age.1 The disease appears to be sex hormone-dependent as symptoms can vary across the menstrual cycle, with pain as a prominent symptom. The condition is also frequently accompanied by dysmenorrhoea and infertility, with the presence of pelvic abdominal lesions. Conservative treatment with anti-inflammatory drugs is often ineffective, and surgical removal of the lesions becomes a viable alternative.2 However, after surgery, the condition can reappear with pain and associated lesions. As the condition is chronic, it can be accompanied by epigenetic changes that may complicate the effectiveness of treatment.3,4
The disease is characterised by the growth of endometrial elements outside of the uterine cavity. Why this occurs is currently unknown, and what factors predispose or contribute to this very common condition in a subpopulation of younger females is not yet evident. It may result from retrograde menstruation,5,6 with attachment and growth of endometrial tissue in this environment. The tissue associated with endometriosis contains blood vessels, nerves, mast cells, myofibroblasts, and macrophages,7-10 and, thus, appears to be a fibrotic lesion that in some respects resembles a progressive scar. As endometriosis tissue can be influenced by sex hormones,11-13 and possibly neuroinflammatory elements,14 it may resemble abnormal scarring such as occurs following a burn injury (hypertrophic scar), or following a traumatic elbow injury leading to a joint contracture.15,16 Thus, the initiating events may differ but the cellular and molecular interactions leading to fibrotic progression may share a number of commonalities. Interestingly, endometriosis has also been linked with risk for other chronic diseases such as asthma,17,18 in which mast cells also play a central role.
PRECLINICAL MODELS
Endometriosis can occur naturally in horses19 and non-human primates,20 and can be induced in rats by auto-transplantation of uterine tissue to the abdomen, or in nude mice by transplantation of human endometrial tissue.21,22 Such models can provide some insights into aspects of endometriosis development and progression, as well as insights into potential interventions, but, thus far, the aetiology of endometriosis in humans and in non-human primates remains undefined.
ROLE OF SEX HORMONES IN ENDOMETRIOSIS
As discussed above, endometriosis is a sex hormone-mediated inflammatory disease. With regard to endometriosis, oestrogen has been implicated in the activity of macrophages,10 fibroblasts and myofibroblasts,13 nerves,10,12 and mast cells.7,23 It is also clear that oestrogen is a regulator of inflammatory processes, and activation of inflammatory cells can contribute to nerve fibre-mediated pain.14 In addition, sex hormones, such as oestrogen, are known to contribute to wound healing in response to injury.24,25 Both oestrogen and progesterone have been implicated in mast cell degranulation.26
After menopause, inflammatory processes decline, wound healing is compromised, and endometriosis in most patients becomes latent.5,27 However, 2–4% of women still continue to experience endometriosis after menopause.5 Therefore, either alternative mechanisms could potentially allow the disease to continue in this subpopulation (e.g. due to epigenetic modifications4,28) or local production of oestrogen could continue to contribute to disease activity in the post-menopausal state.
While endometriosis is considered oestrogen-dependent, in part based on menstrual cycle dependency, a role for progesterone is also likely.29 Furthermore, the impact of sex hormones could be a secondary sequela of hormone level changes (e.g. water retention with increased turgor of endometrial lesions contributing to pain).
REGULATION OF PROTEINASE EXPRESSION IN ENDOMETRIOSIS
Role of Sex Hormones and Their Receptors
Proteinases such as tissue factor,30 elements of the fibrinolytic system,31 cathepsins,32 mast cell tryptase and chymase,33 and matrix metalloproteinases (MMP)34 have all been implicated in endometriosis. As these enzymes can facilitate extracellular matrix turnover, activate pro-enzymes and other molecules, and serve other functions related to inflammation, coagulation, collagen deposition, and angiogenesis, they are likely central to endometriosis progression. In this regard, their role is likely not different from their roles in other fibrogenic processes and wound healing.
Much research has focussed on the MMP, their expression in endometriosis, and their regulation by progesterone35,36 and oestrogen.34 Progesterone appears to limit MMP expression,35,36 while oestrogen is reported to enhance MMP-9 expression,37 and MMP-9 levels are enhanced in endometriosis.38 As oestrogen functions primarily via estrogen receptor (ER)-alpha and ER-beta in cells, receptors known to be expressed in endometrial tissue,39,40 this response is potentially contributing to the symptoms of endometriosis and its progression. However, the role of oestrogen receptors in endometriosis is somewhat controversial,39,40 with ER-beta a more prominent variant in endometriosis than normal tissue.39
Interestingly, oestrogen receptors in the absence of oestrogen can also regulate expression of some MMP41-43 and these include MMP-1 and MMP-13. The addition of oestrogen actually depressed expression rather than enhancing expression. ER-beta was more effective than ER-alpha in many of these responses, and, interestingly, genetic variants of the MMP-1 promoter region were also differentially regulated. Some of these same variants, as well as those for MMP-2, 7, and 9 were found to be associated with risk of endometriosis.44 Intriguingly, splice variants of ER-beta that do not bind oestrogen were also effective in upregulating expression of MMP-1,45 and splice variants have also been detected in endometriosis tissues.46
MAST CELLS IN ENDOMETRIOSIS
Mast cells, often thought of in the context of allergies or asthma, are present in most tissues throughout the body, including connective tissues and many organs.7,15,16 Mast cells are also very prevalent in normal and abnormal healing environments, with elevated numbers, in abnormal healing conditions.7,15,16 Mast cells are very prevalent in endometriosis tissue, and many of them appear to be activated and degranulated.47-49 In addition, many of the mast cells were localised very close to neural elements,49 and, as such, could play an active role in neuroinflammatory processes.7,15,16 We have previously seen neural elements ending in normal dense connective tissues very close to a mast cell, implying that nerve-mast cell co-localisation may also play a role in normal tissue functioning as well.7,15,16
Mast cells and their products could contribute to several features of endometriosis. Mast cell tryptase can activate protease activated receptors (PAR), particularly PAR-2.50 PAR are known to be expressed in endometrium and endometriosis.51 Activation of PAR-2 on cells may participate in pain processing52 and angiogenesis.53 Mast cell tryptase can also activate myofibroblasts and contribute to fibrosis.15,16 Histamine released from mast cells has been studied for decades and has multiple activities, including enhancing tissue oedema and many other effects. Activated mast cells can also release a number of pro-inflammatory cytokines, mediators, and growth factors, and thus, can have a very potent and varied impact on a target tissue, particularly one that appears to be abnormally regulated, as in endometriosis. The enhanced presence of mast cells, many of which appear to be activated, in endometriosis tissue has led to the proposal that mast cells should be targeted with drug interventions to assist in controlling endometriosis progression and symptoms.7,23,54,55 Based on the findings that activated mast cells are present in endometrial tissues, oestrogen/progesterone can influence mast cells, mast cells can release many biologically active molecules, and mast cell numbers are increased in endometrial tissues, this cell may be an excellent cell to target in endometriosis, alone or in combination with other targets.
MAST CELL STABILISERS
Mast cell stabilisers are drugs which inhibit or prevent mast cell degranulation. These include two drugs, ketotifen and sodium cromoglycate, that have a long history of use in the treatment of asthma. While ketotifen is now off patent protection, it has been used safely for >40 years in adult and paediatric populations. It is not totally targeted specifically for mast cells, and is also reported to inhibit degranulation of polymorphonuclear leukocytes.17,18 Thus, with the development of new potential indications (e.g. endometriosis), there may be an impetus for industry to develop newer versions that may be more specific for mast cells, or more active in specific diseases or conditions.
USE OF MAST CELL STABILISERS IN ABNORMAL FIBROPROLIFERATIVE CONDITIONS
Abnormal healing follows induction of skin injuries in the red Duroc pig56,57 and following trauma and immobilisation of a knee injury in a rabbit model.58 The former exhibits characteristics of hypertrophic scarring and also fibrogenic scarring.59 In the red Duroc pig, this abnormal scarring response appears to have a genetic component.60 The rabbit model exhibits cellular and molecular characteristics of joint contractures that occur in a subset of humans who experience an elbow injury.15,16
In these two models, elevated numbers of mast cells, nerves, and myofibroblasts have been observed leading to the conclusion that the cells contribute to fibroproliferative dysfunction. These cells have been postulated to form a cellular ‘axis’ (Figure 1) in which the mast cell plays a central role.7,15,16 In this proposed axis, the fibroblasts and myofibroblasts are the effector cells which release fibrotic molecules, such as collagens, and contract the fibrotic matrix. Neuropeptides from nerves or, in the case of endometriosis, possibly sex hormones, impact mast cells, which then release molecules that enhance the activity of fibroblasts and myofibroblasts. Thus, the mast cells function as accelerators or amplifiers of the fibrotic environment. This axis is postulated to be dysfunctional in the two models, but whether the initiator of the dysfunction is only due to the nerves (e.g. neuroinflammation), or some other cell type or stimulus, remains to be determined. Treatment of skin wounds on red Duroc pigs with the asthma drug ketotifen from the time of injury prevented development of the abnormal fibrogenic response to injury and led to a more typical healing response.57
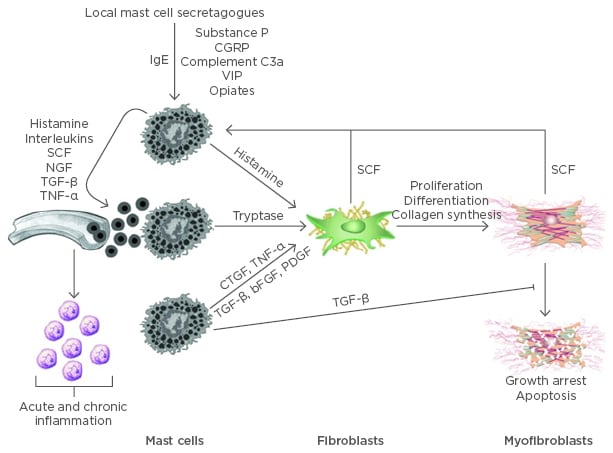
Figure 1: Mast cells mediated inflammation and fibrosis.
Mast cells circulate as CD34-positive precursor cells and terminally differentiate in connective tissues. Both IgE-dependent and independent mechanisms can activate mast cells causing the release of preformed and newly synthesised pro-inflammatory mediators. Many of these mediators increase vascular permeability and promote the recruitment of other inflammatory cells and additional mast cell precursors. SCF is also secreted by activated fibroblasts and myofibroblasts, further potentiating mast cell recruitment and proliferation. TGF-β is a potent fibroblast mitogen and stimulator of myofibroblast differentiation. It also impedes myofibroblast apoptosis.
bFGF: basic fibroblast growth factor; CGRP: calcitonin gene-related peptide; CTGF: connective tissue growth factor; IgE: immunoglobulin E; NGF: nerve growth factor; PDGF: platelet-derived growth factor; SCF: stem cell factor; TGF-β: transforming growth factor beta; TNF-a: tumor necrosis factor alpha; VIP: vasoactive intestinal peptide.
Adapted from Monument et al.16
If treatment was delayed until 28 days post-injury, the drug was without effect. Stopping the drug treatment after epithelisation of the wounds did not lead to a reactivation of the abnormal fibrogenic response. Thus, this mast cell stabiliser appeared to exert its influence early in the response to injury. Interestingly, treatment with ketotifen led to a decline in detectable nerves, mast cells, and myofibroblasts in the skin scar tissue, which likely also indicated that the postulated axis was not unidirectional and interfering with mast cell degranulation impacted other elements of this axis. Finally, treatment of skin wounds with ketotifen in Yorkshire pigs that healed normally was without effect and did not influence the number of cells in the axis that could be detected. Thus, the drug did not appear to influence normal healing of skin wounds.
Similar studies in the rabbit model of post-traumatic joint contractures using ketotifen treatment also led to improvement in the healing process and significant declines in the extent of the joint contractures (˜50%), as well as cells of the nerve–mast cell–myofibroblast axis.61,62 The suggestion that ketotifen was likely inhibiting mast cell degranulation, arose from recent studies demonstrating that serum levels of mast cell tryptase were significantly depressed in the treated rabbits.63
The mast cell stabiliser sodium cromoglycate has also shown effectiveness in a rat model of experimental endometriosis.55 In this model, transplantation of endometrial tissue to the abdominal wall followed by a 2-week treatment regimen with sodium cromoglycate led to significant declines in activated mast cells, as well as tissue levels of mast cell tryptase and serum levels of tumour necrosis factor alpha. However, the size of the lesions was not apparently influenced by the treatment. As myofibroblasts have also been identified in endometriosis lesions,9 as well as nerves,49 it would be of interest to assess the influence of mast cell stabiliser treatment of the transplanted tissues on levels of these two cells as well, particularly since mast cells and nerve elements have been detected in close proximity in human endometriosis tissues.49 Interestingly, close proximity of nerves and mast cells have been noted in unrelated tissues, and it appeared that neuropeptides released from nerves could impact the mast cells (Figure 1), leading to an amplified impact on the target tissue.
From our studies using ketotifen,57,61,62 and those of Zhu et al.55 with sodium cromoglycate, inhibition of mast cell degranulation may be a viable direction to explore. In contrast, D’Cruz and Uckun54 proposed using a janus kinase (JNK) 3 inhibitor, JANEX-1, and recently a JNK inhibitor has been reported to cause regression of endometriotic lesions in rodent models.64 While targeting mast cells using these approaches has not yet led to clinical trials in endometriosis, a clinical trial is currently underway using ketotifen to prevent joint contracture development following traumatic elbow injuries.65
Are There Commonalities in Dysregulated Fibrogenic Responses to Inflammation and Injuries?
Joint contracture development following a trauma to the elbow occurs in 10–15% of those injured.15,16 Development of a contracture is accompanied by a fibrotic response in which the healing process is dysregulated and does not proceed through to maturation and remodelling of the scar. In many cases, the patients undergo surgery to release the contracture, but often with only partial recovery.15,16
Similarly, development of a hypertrophic scar after a severe burn injury is also a fibrotic response to the injury, with excessive matrix deposition, myofibroblasts, and mast cells. For many patients, the hypertrophic scars have to be surgically removed. Interestingly, female sex is also a risk factor for hypertrophic scar development.66 The abnormal fibrogenic skin wound healing, in the previously discussed porcine models, also exhibits some similarities with hypertrophic scarring.
Given these similarities, perhaps much of the uniqueness of endometriosis is associated with where the abnormal fibrotic tissue is located, its sex hormone dependency, and the sequelae of the fibrosis, rather than any intrinsic uniqueness in the cellular aspects of the dysregulated processes contributing to progression and chronicity. Thus, some of what has been learned from these other diseases or conditions, as well as from the preclinical models discussed, could provide insights into focussing research directions forward with mast cells as a target.
Future Directions for Assessment of Efficacy and Implementation of Mast Cell Stabilisers in Patients with Endometriosis
To undertake a clinical trial of mast cell stabilisers, such as ketotifen or sodium cromoglycate, in populations of young females with endometriosis, investigators must proceed with caution as many of these individuals are of reproductive age, and mast cells have been implicated in normal ovulation events and others related to reproduction in some species.67 However, the complexity of endometriosis in patient populations could be approached in phases to strengthen the link between mast cell activity and symptoms and then identify those best suited to participate in pilot trials using the mast cell stabilisers (Table 1).
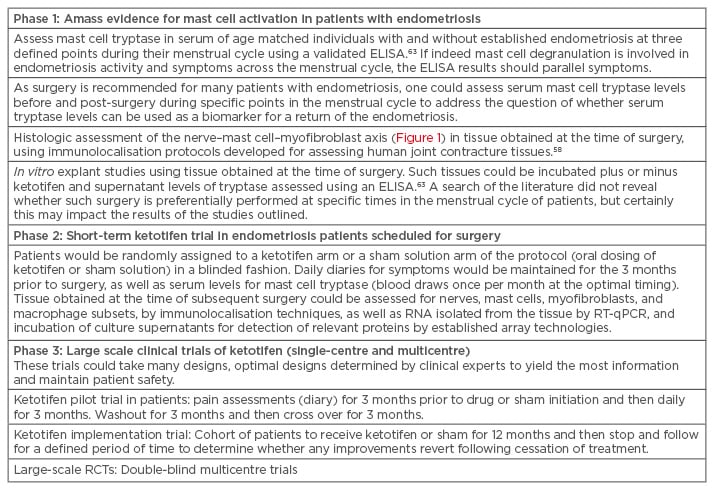
Table 1: Potential clinical pathway to confirming mast cells as a therapeutic target in endometriosis.
ELISA: enzyme-linked immunosorbent assay; RCT: randomised controlled trial; RT-qPCR: quantitative reverse transcription polymerase chain reaction.
One of the current gaps in endometriosis research is a lack of good biomarkers, such as serum components, that can be used to monitor disease activity, assess the impact of interventions, or assess the return of disease activity following surgery prior to overt symptoms being evident. Serum mast cell tryptase levels, as assessed by an enzyme-linked immunosorbent assay63 could contribute to filling this gap.
Finally, it is clear that epigenetic changes occur during progression of endometriosis.3,4,28 These changes (DNA methylation, histone modifications, and alterations to miRNA profiles) can lead to alterations in cell responsiveness to interventions. In some chronic diseases (e.g. rheumatoid arthritis), these changes can occur in fibroblasts and other cells. Thus, endometrial lesions early in the disease may respond differently to targeting mast cells than those with more advanced disease. Such factors may need to be considered when assessing effectiveness.
CONCLUSIONS
Based on the above discussion, there is considerable circumstantial evidence to support the use of mast cell targeted drug interventions in the treatment of endometriosis. There is an advantage for using known drugs such as ketotifen and sodium cromoglycate, both with long track records of use for the treatment of asthma in a variety of populations. Thus, their safety and efficacy is well documented. However, repurposing these drugs is a viable approach, but one that will require a systematic analysis in patient populations (Table 1). Given the large population of females with endometriosis (5–15% of females worldwide), and the impact of the condition on these young women, new approaches that could impact their quality of life should be entertained. However, this approach using mast cell targeted drugs may address the how of the disease, but not why the disease occurs, so it would only be a stop-gap approach until new information arises as to why it develops, and what is unique about the subset of women who experience the disease (e.g. genetics, epigenetics, exposure to environmental stimuli, and stochastic events).








