Abstract
Human oocytes have an abundance of mitochondria that have their own genome. Mitochondrial functions are exerted through evolutionarily-developed interactions between the nucleus and mitochondria. Since 1996, fertility clinics have practiced various types of germline mitochondrial DNA (mtDNA) modification that alter the composition of mtDNA copies in oocytes or zygotes using micromanipulation. Experimental reproductive medicine has primarily intended to treat intractable infertility and has been used to prevent the maternal transmission of a pathogenic mtDNA mutation to offspring. In some cases, it has helped parents have a healthy genetically-related child; in others, it has resulted in miscarriages, aneuploid fetuses, or developmental disorders in the offspring. Adverse events have raised ethical controversy, leading to restrictive or prohibitive policies in the USA and China. Conversely, the UK recently became the first nation to explicitly permit two types of germline mtDNA modification (termed mitochondrial donation) for the sole purpose of preventing serious mitochondrial disease in offspring. The aim of this review is three-fold: first, to reshape the medical concept and evolution of germline mtDNA modification, while revisiting 14 clinical cases. Second, to analyse the legality of mtDNA modification, focussing on 16 Western countries. Finally, to consider the ethical aspects, including permissible cases, reproductive options, use of preimplantation and prenatal testing, and the humane follow-up of resultant children. The clinical use of germline mtDNA modification will likely become legal, at least for use in preventative medicine, in some countries. However, the potential clinical, ethical, and evolutionary implications mean that caution is required when considering its wider application.
INTRODUCTION
The majority of human cells have two genomes: nuclear DNA (nDNA), with approximately 24,000 protein-coding genes, and mitochondrial DNA (mtDNA), with only 13 protein-coding genes. Mitochondria are small organelles that exist in the cytoplasm and are involved in various cellular functions. The production of ATP through the respiratory chain is one of the most important functions of the organelles. Mitochondrial functions are exerted through the co-ordinated expression of genes in mtDNA and nDNA, which have become highly specific over evolutionary time. Regarding human mtDNA, a spermatozoon has 100–1,500 copies of the organelle genome, whereas a mature oocyte has as many as 200,000–300,000 copies of mtDNA.1 Paternal mitochondria are specifically digested after fertilisation; as a result, only maternal mtDNA is transferred to the offspring. Mutations to the 13 protein-coding mtDNA genes have been linked to various forms of human mitochondrial disease.2 Although POLG in the nDNA, which encodes the catalytic subunit of mitochondrial DNA polymerase, has been suggested to be associated with infertility, mtDNA genes that only cause infertility remain elusive.3,4
From the 1980s to the early 2000s, rodent experiments have demonstrated the feasibility of altering the cytoplasm of oocytes (ooplasm) by cytoplasmic transfer. Soon after, it was demonstrated that the cytoplasm of embryos can be largely replaced by transferring a karyoplast (nuclei [or a nucleus] with a plasma membrane containing a small amount of cytoplasm) to a different enucleated zygote.5-7 Such outcomes led to the development of reproductive medicine involving a cytoplasmic or karyoplast transfer that alters the composition of mtDNA copies in oocytes or zygotes. In 1996, a clinic in the USA initiated ooplasmic transfer (OT), and reported the birth of a baby in 1997; this is believed to be the first case of human germline genetic modification.8,9 Subsequently, some OT cases have helped prospective parents have a genetically-related child, whereas others have resulted in miscarriages, aneuploid fetuses, and the onset of a developmental disorder in the offspring.10,11 In 2003, a collaboration between a Chinese group and a team from the USA reported the first pronuclear transfer (PNT), which was performed with the intention of largely replacing the cytoplasm of a patient’s zygote with that of a donor zygote.12 The PNT performed in China led to a triplet pregnancy; however, two fetuses died after selective fetal reduction. Such adverse events have led to restrictive or prohibitive regulatory policies in the USA and China.13 Conversely, in 2015, the UK legalised PNT and maternal spindle transfer (MST), which can largely replace ooplasm, for the sole purpose of preventing serious mitochondrial disease in offspring.14 In 2017, the first MST procedure performed by researchers from the USA and Mexico led to the birth of a healthy baby.15
With the current climate concerning mtDNA modification in mind, this article first reviews the medical concept and evolution of germline mtDNA modification, while revisiting 14 clinical cases. Next, the legality of the procedures is analysed, focussing on 16 Western countries, because an international treaty in the biomedical field was established in Europe.16 Furthermore, ethical aspects are considered regarding permissible cases, reproductive options, the use of preimplantation genetic diagnosis (PGD), and prenatal testing and humane follow-up of resultant children.
MEDICAL CONCEPT AND EVOLUTION
Table 1 shows 14 clinical cases of germline mtDNA modification that have been performed in nine countries. Eleven reports were published from 1997–2003. The remaining three reports were published within the last 3 years, after a decade-long period without relevant publications.
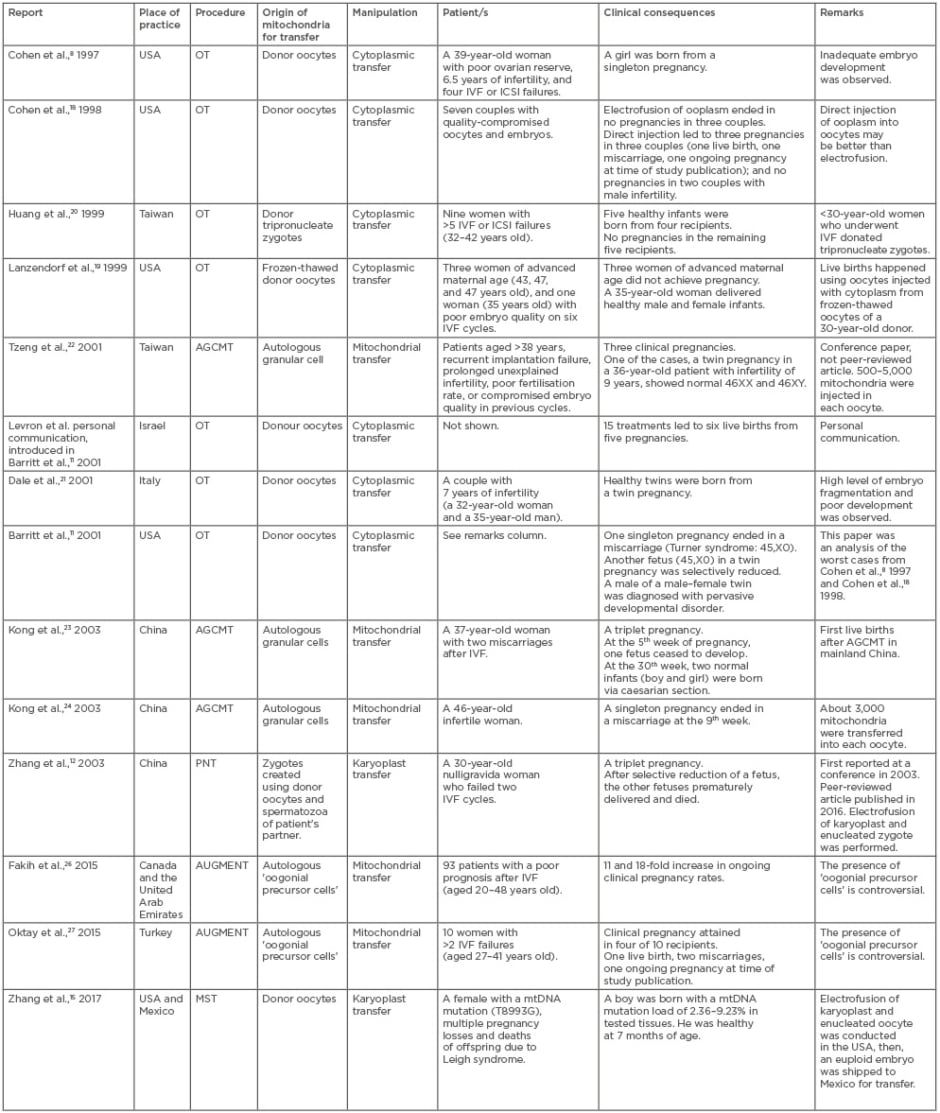
Table 1: Clinical implementations of germline mitochondrial DNA modification.
AGCMT: autologous granular cell mitochondrial transfer; AUGMENT: autologous germline mitochondrial energy transfer; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; PNT: pronuclear transfer; OT: ooplasmic transfer.
The Beginning of Germline mtDNA Modification
In 1996, a USA clinic initiated a clinical study of OT, in which 5–15% of ooplasm aspirated from mature oocytes donated by fertile women was injected into mature oocytes of infertile patients, along with a spermatozoon.11 The subjects included 33 infertile women who had experienced repeated implantation failure and poor embryo development after in vitro fertilisation (IVF).17 Based on a hypothesis that IVF failures could be due to cytoplasmic deficiency rather than aneuploidy in nDNA, the study intended to enhance the developmental potential of the patient’s embryos. In 1997, a girl was born via OT (Table 1).8 mtDNA typing showed sustained heteroplasmy representing both donor and recipient mtDNA in the clinical specimen, suggesting that heteroplasmic mitochondrial populations persist and may be replicated during development (Table 1).9 Likewise, other OT cases intended as infertility treatment for women with a history of implantation failure and/or poor embryo development in women of ≥35 years of age can be found in Table 1. In typical OT, ooplasm from a fresh, mature oocyte donated from a fertile woman is transplanted into the oocytes of an infertile patient through intracytoplasmic sperm injection because electrofusion of the ooplasm and oocytes likely damages the viability of the resultant oocytes.18 OT variants in the USA and Taiwan used frozen-thawed donor oocytes and donor tripronucleate zygotes as a source of ooplasm.19,20 These efforts led to live births in some cases.8,10,11,19-21 Aneuploidy, namely 45,X0 (Turner syndrome), was found in two different fetuses in the USA after OT, which resulted in a miscarriage and selective fetal reduction (Table 1). Furthermore, 1 of 17 children born via OT in the USA was diagnosed with a borderline pervasive developmental disorder (Table 1).10
Autologous Mitochondrial Transfer
Autologous granular cell mitochondrial transfer (AGCMT) does not depend on oocyte donation. In the three AGCMT cases from Taiwan and China, hundreds to thousands of mitochondria from the patient’s own granular cells were injected into quality-compromised oocytes (Table 1).22-24 Importantly, although AGCMT adds the patient’s mitochondria to their own oocytes, it can potentially induce heteroplasmy in the injected oocytes by mixing mitochondria from somatic cells and germ cells in one individual.25 AGCMT has led to live births as well as a fetal death and miscarriages. In 2015, two clinical reports from Canada, the United Arab Emirates, and Turkey reported the effects of autologous germline mitochondrial energy transfer (AUGMENT) on clinical pregnancy rates.26,27 AUGMENT, which appears to be a derivative of AGCMT, uses mitochondria from the patient’s oogonial precursor cells. However, the populations of the two studies included younger women of 20–27 years of age (Table 1). Furthermore, their study design, as well as the presence of oogonial precursor cells in older women, is controversial.28-30
Karyoplast Transfer
The first PNT implementation reported from China in 2003 intended to treat intractable infertility via karyoplast transfer using a larger micropipette12 (30–40 µm, 5–6-times larger than the needle used in intracytoplasmic sperm injection) (Table 1, Figure 1B). The subject was a 30-year-old woman who experienced embryo arrest in infertility treatment; she had received two IVF cycles prior to PNT. PNT led to a triplet pregnancy; however, after selective fetal reduction, one of the fetuses died of respiratory distress and the other of cord prolapse. Despite a lack of detailed data, the report claimed that the karyotypes of the fetuses were normal, that the nDNA of the fetuses and the patient matched, that the mtDNA profiles of the fetuses and donor were identical, and that the patient’s mtDNA was not detected in the fetuses. In PNT, electrofusion was performed to fuse the patient’s karyoplast with an enucleated zygote, which differed from the technique in the USA OT study (Table 1).18
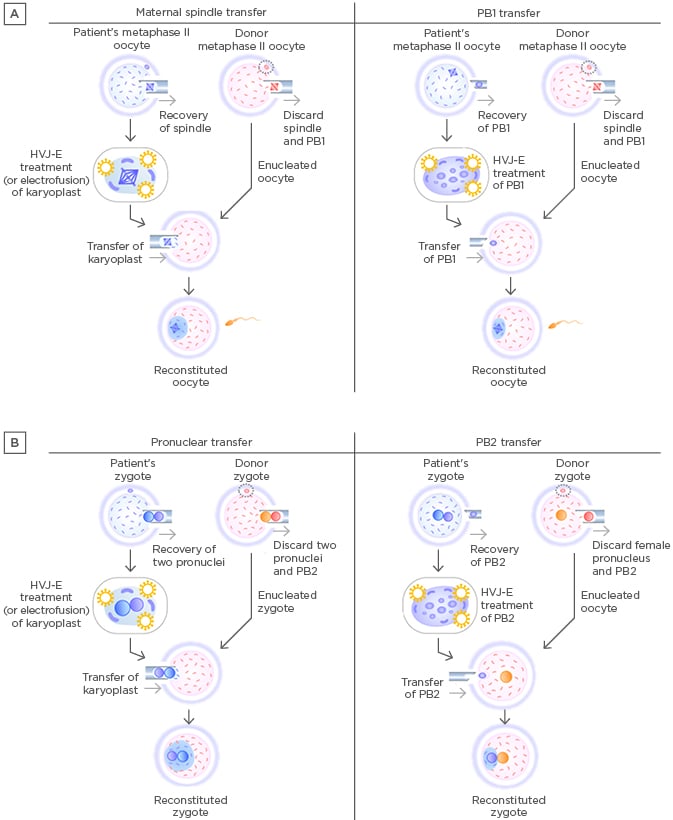
Figure 1: Procedures of maternal spindle transfer, first polar body transfer, pronuclear transfer, and second polar body transfer.
A) Procedures of maternal spindle transfer (left) and PB1 transfer (right). B: Procedures of pronuclear transfer (left) and PB2 transfer (right).
HVJ-E: haemagglutinating virus of Japan envelope; PB1: first polar body; PB2: second polar body.
In 2017, a group led by the first author of the 2003 PNT report12 published the first report on MST in a cross-border project between the USA and Mexico (Table 1, Figure 1A). MST differed from previous germline mtDNA modifications in that it used karyoplast transfer in oocytes to prevent the onset of mitochondrial disease (specifically Leigh syndrome) in offspring. The female subject had experienced miscarriages and the loss of offspring due to an ATPase gene mutation in her oocyte mtDNA. The mtDNA mutation load of the woman’s oocytes was almost 100%. The mtDNA haplogroup of the patient and the oocyte donor were different (I and L2c, respectively). The heteroplasmy level in the blastocysts after MST was 5.7%, which was higher than the levels in other preclinical reports using human oocytes (undetectable or <1%).31,32 This MST case led to the birth of a boy. However, the mtDNA mutation load of his tested tissues varied from 2.36–9.23%, and his long-term prognosis remains unclear because the reversal of a pathogenic mtDNA copy may happen.33,34
Other Procedures
In addition to PNT and MST, two types of karyoplast transfer have been proposed: germinal vesicle (GV) and aggregated chromosome transfer. GV transfer removes and transfers the nucleus surrounded by the membrane in oocytes in the prophase of meiosis I.35 Aggregated chromosome transfer is performed from the breakdown of the GV to the formation of the metaphase-I spindle, during which chromosomes are visible.36 However, both procedures have not yet been used clinically.
More recently, newer germline mtDNA modification procedures have been proposed: first polar body transfer (PB1T) and second polar body transfer (PB2T).37,38 In PB1T, a first polar body is transferred to an enucleated mature oocyte (Figure 1A). In PB2T, a second polar body is removed from a zygote and replaced with the female pronucleus in a donor zygote (Figure 1B). Polar body transfer may have advantages over MST and PNT in terms of mitochondrial carry-over because human polar bodies contain few mitochondria.39 However, fusion of a polar body and karyoplast requires haemagglutinating virus of Japan-envelope treatment, the safety of which remains unknown in human reproduction. The histories of PB1T and PB2T are shorter than the histories of PNT and MST. Despite the successful production of mice using first or second polar bodies,40 human reproduction involving polar body transfer is still a long way from clinical application; further research is required to ensure the safety of the resultant offspring.
The history of germline mtDNA modification began with the clinical use of OT in 1996. These initial techniques gave rise to variants, including autologous mitochondrial transfer in oocytes and karyoplast transfer in zygotes and oocytes. However, the characterisation of the mitochondrial functions and mtDNA profiles in patients and the resultant offspring was largely insufficient in such small-scale studies. Following the first MST procedure, the heteroplasmy levels of the patient and her baby were analysed; however, the rate of mtDNA carry-over was relatively high in the offspring. Low levels of heteroplasmy can lead to subsequent reversal of the original mitochondrial genotype in MST.33,34 It is hypothesised that mtDNA haplotypes with specific D-loop polymorphisms are preferentially amplified, potentially causing the reversal.34 Additionally, the need for matching between nDNA and mtDNA in MST and PNT is controversial. Some assert that mismatching between donor mtDNA and patient nDNA might cause dysfunctional respiratory chain,41 while others disagree.33,34,42 Thus, germline mtDNA modification that intervenes in evolutionarily-developed mitochondrial–nuclear interactions using micromanipulation remains largely experimental in human reproduction.
LEGALITY IN THE WESTERN WORLD
Although adverse events following OT and PNT for infertility treatment led to prohibition of germline mtDNA modification in the USA and China, the UK became the first nation to permit PNT and MST, for the sole purpose of preventing serious mitochondrial disease in offspring. In Europe, the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine (ETS No. 164) was concluded in 1997 (the so-called Oviedo Convention).16 This treaty, which is the only binding international law in the biomedical field, stipulates that “An intervention seeking to modify the human genome is only to be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants” (Article 13).16 Since the Oviedo Convention appears to prohibit germline mtDNA modification for human reproduction, it is worth analysing the legality of germline mtDNA modification focussing on the Western world. Sixteen countries were selected based on observed activities, including clinical reports, trial registries, advertisements relevant to germline mtDNA modification.13 Of the 16 countries, 10 ratified the Oviedo Convention; Germany, Italy, Northern Cyprus, Russian Federation, the UK, and Ukraine did not (Table 2).16
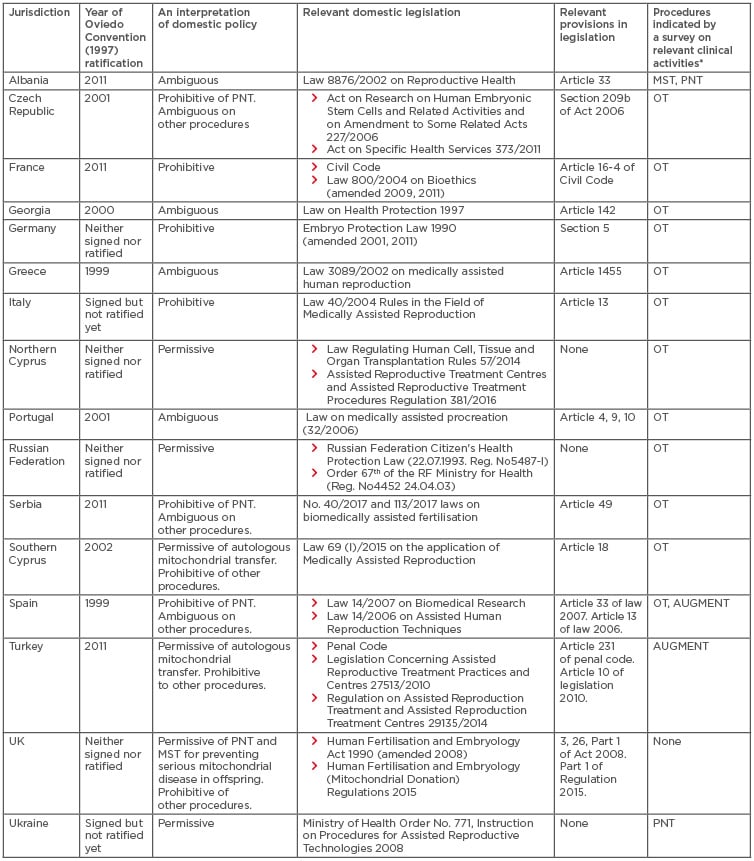
Table 2: The policies regarding germline mitochondrial DNA modification in 16 countries.
*Sixteen countries were selected based on the survey regarding germline mtDNA modification-relevant reports, trial registries, and advertisements on clinic websites or medical tourism websites.13
AUGMENT: autologous germline mitochondrial energy transfer; OT: ooplasmic transfer; MST: maternal spindle transfer; mtDNA: mitochondrial DNA; PNT: pronuclear transfer.
The domestic policies relevant to germline mtDNA modification in the 16 countries were further analysed (Table 2). France, Germany, and Italy legally prohibit mtDNA use in reproductive medicine. Conversely, Northern Cyprus, the Russian Federation, and Ukraine, are permissive to its use in reproductive medicine. In the remaining 10 countries, the UK maintains the legal prohibition of all germline mtDNA modifications except PNT and MST for disease prevention (use for infertility treatment is illegal). Southern Cyprus and Turkey only permit autologous mitochondrial transfer, such as AGCMT and AUGMENT. Domestic laws in the Czech Republic, Serbia, and Spain only prohibit PNT; the legality of other procedures is ambiguous. The legality of germline mtDNA modification in Albania, Georgia, Greece, and Portugal is ambiguous because, despite their ratification of the Oviedo Convention, these countries appear to allow its use in reproductive medicine.
Thus, there is some ambiguity regarding the domestic legality of germline mtDNA modification in Southern Cyprus, Turkey, Czech Republic, Serbia, Spain, Albania, Georgia, Greece, and Portugal, which ratified the Oviedo Convention. The Oviedo Convention stipulated that “Each Party shall take in its internal law the necessary measures to give effect to the provisions of this Convention” (Article 1).16 However, OT and AUGMENT are advertised on the internet and may be offered in those countries (Table 2). These findings suggest that these nine countries have delayed or neglected amending or enacting relevant regulations prohibiting germline mtDNA modification, as others suggest.43 There are inherent legal issues surrounding Article 13 of the Oviedo Convention, which prohibits the introduction of “any modification in the genome of any descendants”, considering the characteristics of germline mtDNA modification. For example, males who undergo germline mtDNA modification do not pass their mtDNA onto the next generation. In addition, there is no specific legal definition of the term genome.16 Some may specifically interpret ‘genome’ to mean nuclear genome.44 In contrast, ‘nuclear DNA’ and ‘mitochondrial DNA’ are used in the UK’s regulations regarding mitochondrial donation. Additionally, some might narrowly interpret Article 13 as the prohibition of modifying a gene(s) in mitochondrial genome of oocytes or zygotes, although germline mtDNA modification changes the composition of the mitochondrial genome copies. Thus, it is suggested that the domestic policies in Western countries and the Oviedo Convention were never meant to regulate germline mtDNA modification.
ETHICAL ASPECTS
Although germline mtDNA modification is permitted or may not be unlawful in some countries, researchers in such countries are required to practice germline mtDNA modification with due consideration of its ethical implications.
Applicable Cases
The history of PGD suggests that germline mtDNA modification will initially be used for disease prevention rather than infertility treatment.45 Moreover, mutations in any of the 13 protein-coding mtDNA genes have been linked with various forms of mitochondrial disease.2 However, the link between genes in mtDNA and infertility is currently controversial.3,4 Potential targets of germline mtDNA modification to prevent mitochondrial disease in offspring include women who have lost children due to mitochondrial disease and women with an inherited mutant gene in their oocyte mtDNA.15,46 mtDNA modification use for such women is understandable as a safeguard against genetic disease in future children.47,48 Although PGD may be used to avoid the birth of children with mitochondrial disease, the selection of embryos or oocytes is not applicable to women who only have oocytes with a high mtDNA mutation load. In addition, PGD that simply selects for the embryo having the lowest heteroplasmy level is unlikely to eliminate the risk of transmitting mtDNA mutations.49 Despite these limitations, in some countries the clinical rationale and assumed welfare of the offspring might justify the use of some germline mtDNA modifications for women with a pathogenic mtDNA mutation in their oocytes who want to protect the future of their children from serious mitochondrial disease.
Reproductive Options
Excluding autologous mitochondrial transfer, the implementation of germline mtDNA modification requires oocyte donation. The direct use of donor oocytes can also help parents protect future children from life-threatening mitochondrial disease.50 Donor oocyte availability suggests that the direct use of donor oocytes as well as germline mtDNA modification can be another reproductive option. Of course, many parents want to use PNT or MST to have a genetically-related child.51 In contrast, some prospective mothers may be satisfied with the genetic relatedness between a resultant child and their partner. In the USA OT study, prospective parents considered the use of oocyte donation.8 Thus, in addition to the experimental nature of germline mtDNA modification, the option of directly using donor oocytes should be explained to prospective parents.
Use of Preimplantation or Prenatal Testing
Prior to the transfer of embryos created via germline mtDNA modification, PGD can identify and exclude aneuploid embryos and embryos with an unacceptable level of heteroplasmy. Notably, PGD requires an additional intervention of cell biopsy, which can damage the viability of embryos.45 This is particularly important when performing radical karyoplast transfer. Indeed, it was reported that physicians who plan to perform PNT in the UK were unwilling to use PGD.52
Instead, prenatal testing using amniotic fluid and chorionic villus sampling can confirm the genetic condition of a resultant fetus; however, invasive prenatal testing is associated with a miscarriage risk (approximately 1/300). Nevertheless, the use of prenatal testing should be carefully discussed because some parents would likely want to know whether germline mtDNA modification has been effective prior to the birth of their child. However, all treatments have risks. Prenatal testing may show that a pathogenic mtDNA mutation was not sufficiently reduced. In doing so, some women may feel distress over the decision of whether to maintain or terminate the pregnancy because they consented to experimental reproductive medicine to prevent their pathogenic mtDNA mutation from affecting their children. Due to the complicated ethics, prior sufficient counselling may be valuable for prospective women with a history of miscarriages or childbirths with mitochondrial disease.
Humane Follow-Up of Resultant Children
After the first MST, follow-up was initially planned until the resultant child reached 18 years of age.15 However, the parents requested that no further genetic testing be undertaken, unless there was a clinical benefit for the child.53 In 2016, Chen et al.17 reported a survey result of 17 teenagers born from 13 couples that had used OT at a clinic in the USA between 1996 and 2001. Twelve of the 13 parents completed a questionnaire, while one parent did not respond to repeated requests. In addition, such parents did not agree to standardised clinical analysis due to a lack of disclosure to their children. Thus, the study ended in limited follow-up and possibly a high risk of bias.
It will likely be difficult to follow-up children born via germline genetic modification. However, when applying it to prevent the onset of mitochondrial disease in resultant children, the health of such children should be monitored. The period of follow-up is the most important question regarding the monitoring of such children.54 The UK’s policy on mitochondrial donation only requires physicians to prepare a follow-up plan for resultant children and parents need not consent to it.55 Therefore, the author of this study argues that there is room for improvement in the UK’s policy. Follow-up for several years, decades, or even across generations may be necessary to confirm whether mitochondrial disease is successfully prevented and that no side effects develop. However, the lack of response from one parent in the OT survey17 suggests that such long follow-up periods might infringe on privacy, dignity, and the welfare of the family. Thus, there may be a clash between clinical requirements and ethical considerations regarding the follow-up period of children born via germline mtDNA modification. Although this article cannot present a compelling solution, it is realistic and acceptable to perform follow-up for additional years after a primary endpoint (e.g., healthy birth) or until the resultant child becomes legally competent to refuse it.56 Regarding the potential health risks in later life or transgenerational health risks, rigorous mouse experiments may provide meaningful evidence in advance because the generation time of mice is approximately 2 years.
CONCLUSION
The success of IVF in the UK has led to the worldwide spread of the technique since its first use in 1978. Likewise, if the first mitochondrial donation in the UK succeeds in preventing mitochondrial disease in a resultant child, the clinical use of PNT or MST will likely become legal, at least for disease prevention, in other countries. It is noteworthy that women who underwent mitochondrial donation experienced implantation failures and miscarriages.45 Once PNT or MST for disease prevention is justified, it may be approved for treating intractable infertility in some countries. However, caution is required in its wider use from clinical, ethical, and evolutionary standpoints.








