Abstract
Endometriosis is characterised by the presence of endometrium-like tissue on the pelvis and other organs. Progesterone resistance due to suppressed progesterone receptor (PGR) expression and action is a general feature of endometriosis and is a cause of endometriosis-associated chronic pelvic pain, infertility, inflammatory disorders, and cancer. It appears that progesterone receptor polymorphisms may not be associated with the susceptibility to endometriosis. On the other hand, PGR expression and activity in target cells is significantly dysregulated in both eutopic and ectopic tissues compared with control endometrium. However, the underlying epigenetic mechanisms for PGR suppression in the eutopic tissue are different from ectopic tissue. The aim of this paper was to present an overview of different aspects of progesterone resistance and its application in endometriosis. Finally, this article also presents a few important, unmet questions related to the failure of progesterone treatment in alleviating clinical conditions in endometriosis.
Key Points
1. Endometriosis affects nearly 10% of females of reproductive age; progestin treatment fails in a large number of these patients, likely due to progesterone resistance.2. Progesterone resistance may be due to suppressed progesterone receptor expression and action and is a cause of endometriosis-associated chronic pelvic pain, infertility, inflammatory disorders, and cancer.
3. There is an urgent need for deeper understanding of the cellular and molecular mechanisms of progesterone resistance in endometriosis, for both ectopic and eutopic tissues, to underpin novel approaches to treatment.
INTRODUCTION
Growth of endometrial tissue outside the uterus, frequently but not exclusively, in the pelvic structures gives rise to endometriotic lesions in the peritoneum (peritoneal endometriosis), the ovary (ovarian endometriosis or endometrioma), and the deep pelvis (deep infiltrating endometriosis), and infrequently in the distant organs.1 According to Sampson’s theory, deposits of viable endometrial cells following their reflux into the peritoneal space via the fallopian tubes during menstruation may adhere and grow, and give rise to endometriosis.2 In an elegant review, Redwine3 challenged this theory and demonstrated, by analysing a large number of parameters, that endometriosis tissue is primarily reflected dissimilarity than similarity with eutopic endometrium in the uterus, including inadequate secretory differentiation in endometriotic cells under progesterone dominance during the luteal phase.
Nisolle and Donnez4 speculated that inadequate secretory maturation in the endometriosis might cause from the reduction in progesterone receptor (PGR). Zeitoun et al.5 and Attia et al.6 observed that 17-β-hydroxysteroid dehydrogenase type 2 (17β-HSD2), the activity of which transforms oestradiol to less potent oestrogen (oestrone) and is stimulated by progesterone in endometrial glands, was significantly reduced in endometriotic tissue during the luteal phase, along with markedly repressed levels of immunoprecipitable PGR throughout the menstrual cycle.
The fact that progestin treatment fails to regress endometriosis in three out of 10 females is also indicative of inadequate machinery of progesterone action in the endometriotic tissue.7,8 These observations were suggestive of the absence of certain responses to progesterone action.
Taking these observations together, a theory of ‘progesterone resistance’ as the mediator of pathogenesis of endometriosis was forwarded in the 2000s. Since then, though more intensely in the last decade, the theory has been under scrutiny.8-12 An overview of this theory and its application in explaining the pathogenesis of endometriosis and its management will be presented in this paper.
PROGESTERONE RECEPTOR
Progesterone is a steroid hormone primarily synthesised by the ovaries and adrenal glands, and also by the placenta during pregnancy. Progesterone, although quintessentially the ‘pregnancy hormone’, also plays an important role in several non-reproductive tissues such as the breast, heart and vascular system, brain, and bones.13 The physiological actions of progesterone in target cells are mediated by its binding to PGRs: classical and non-classical. Typically, classical PGR regulates the expression of progesterone responding genes, which result in slowly emerging, long lasting cellular responses. On the other hand, progesterone binding to non-classical PGR activates secondary messengers and signal transduction pathways and mediates rapid responses. A detailed discussion on the physiology of different types of PGR is beyond the scope of the present paper; however, the authors present a synopsis on the topic in the following section. Interested readers may be referred to the comprehensive review articles that covered different aspects of PGR in mammalian cells and specifically in endometrium.11,14-19
Classical Progesterone Receptors
There are two main isoforms of classical PGR: PGR-A (94 kDa) and PGR-B (120 kDa). Both are transcribed from the same gene, but by two different promoters. As schematically presented in Figures 1 and 2, these two isoforms are very similar except that PGR-A lacks 164 amino acids that are present at the N-terminus of PGR-B. Unbound PGR in cytoplasm are complexed with chaperone proteins.14-19 Several lines of evidence suggests that PGR-A is functionally distinct from PGR-B, and thus tissue-specific distribution patterns of PGR-A and PGR-B result in the observed diversity of progesterone- mediated actions.
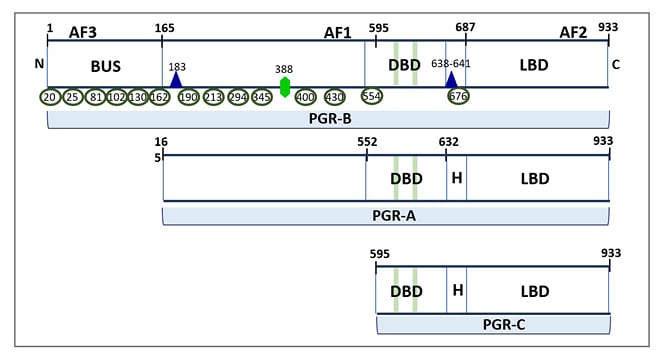
Figure 1: Three isoforms of classical progesterone receptors.
PGR-B (120 kDa), but not PGR-A (94 kDa), includes 164 additional amino acids in the NTD (shown as BUS), where the AF3 domain and multiple phosphorylation sites are located. The NTD also contains an activation factor domain (AF1), which is common for PGR-B and PGR-A. The protein tertiary structure results in a folding at the H region between the DBD and LBD. The green bars in the DBD represent zinc-finger motifs. Post-translational phosphorylation (shown as green ellipses), acetylation (shown as violet triangle), and SUMOylation (shown as green hexagon) can occur basally or in response to ligand binding and affect PGR transcriptional activity. The numbering reflects amino acid residue positions.
AF1: first activation factor; AF2: second activation factor; AF3; third activation factor; BUS: B-upstream segment; DBD: DNA-binding domain; LBD: ligand-binding domain; NTD: N-terminal domain; PGR: progesterone receptor.
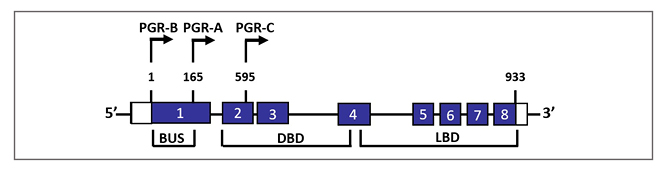
Figure 2: Schematic representation of genomic configuration of three classical progesterone receptor isoforms and splice variants.
The nuclear PGR gene is composed of eight exons with 3100-bp coding regions and 5’- and 3’-untranslated regions. PGR-B and PGR-A isoforms are transcribed from two alternate transcription initiation sites and are identical to amino acids 165–993. PGR-C (60 kDa) isoform results from an in-frame initiation of translation and lacks exon 1.
BUS: B-upstream segment; DBD: DNA-binding domain; LBD: ligand-binding domain; PGR: progesterone receptor.
Generally, PGR-B is the positive regulator of the effects of progesterone, while PGR-A serve to antagonise the effects of PGR-B.14 When progesterone binds to the ligand-binding domain of PGR, the receptor initiates a series of conformational changes, and it is released from the chaperone proteins to finally enter into the nucleus. In the nucleus, PGR dimerises to form homodimers (AA, BB) or heterodimers (AB), and binds to the progesterone response element sequence in the target gene.14-19 A third variant of PGR, PGR-C isoform (60 kDa), has also been described in humans (Figures 1 and 2). PGR-C also can form heterodimers with PGR-A and PGR-B and regulates their transcriptional activity.20 Such diverse possibilities of dimerisation of PGR potentially gives rise to a wide variety of physiological responses. The binding of PGR dimer to progesterone response elements follows their recruitment to coregulators (coactivators or corepressors) and regulation of the subsequent PGR-mediated target gene expression in an isoform- specific manner.14,19
Figure 3 shows a schema of canonical mechanism of PGR action. PGR isoforms interact with one another and mutually regulate their own activity. For example, PGR-A inhibits PGR-B action by its inhibitory domain and, thereby, PGR-A decreases the effects of progesterone on its target cells.14,19 Additionally, tissue-specific coregulator expression along with subnuclear localisation of PGR and coregulator association may mediate tissue-specific progesterone action.14,19,23
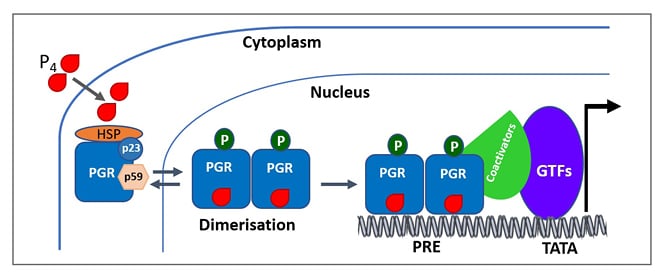
Figure 3: A simplified scheme of the mechanism of progesterone receptor activation.
Ligand-free PGRs are present as inactive complexes associated with HSPs and chaperone proteins (p23 and p59). When progestin binds to the PGR, it undergoes conformational changes along with dissociation of HSPs and chaperone proteins (p23 and p59). PGRs then undergo dimerisation and bind to the HRE in the target DNA. Ligand-dependent conformational changes allow for the recruitment of cofactors and other GTFs to the promoter, producing a transcriptionally active complex that can direct gene transcription.
Adapted from Mani S, Portillo W.21 and Hill KK et al.22
GTF: general transcription factors; HRE: hormone response element; HSP: heat shock protein: P4: progesterone; PGR: progesterone receptor; PRE: progesterone response element.
Non-classical Progesterone Receptors
Non-classical PGRs are usually located on the cell surface as single transmembrane receptors. These receptors belong to G protein-coupled receptor superfamily and are associated with tyrosine kinase activity. Non-classical PGRs are of two types: membrane progestin receptors (mPR) family, also named the progestin and adipoQ receptor (PAQR), and the progesterone receptor membrane component (PGRMC) family, both having several subtypes.8,11,16,19,24 There is evidence to suggest that both types of non-classical PGRs may interact to mediate their actions in target cells. The physiological significance of non-classical PGRs in the uterus is not clear. However, as discussed in the following section, these receptors are associated with the menstrual phase specific functions of uterine cells.8
Progesterone Receptors in Endometrium
Both classical and non-classical PGRs exhibit differential expression depending on endometrial cell type, and the phase of the menstrual cycle (Figure 4). Both PGR-A and PGR-B are expressed in the endometrial epithelium during early to mid-luteal phase, seemingly in preparation of embryo implantation.18,19 The inhibitory effect of PGR-A on the expression of PGR-B causes negative regulation of the action of PGR-B, and thereby promotes hyperplasia and inflammation in this tissue.18,19 During implantation, however, the expression level of PGR-A drops, while that of PGR-B remains constant to support the secretory function of glands of endometrium functionalis. On the other hand, PGR-A is the dominant isoform in endometrial stromal cells throughout the luteal phase and provides support to decidualisation, which is integral to the implantation process.16-19 In fact, very low expression of both isoforms in endometrial cells may result in unexplained infertility and implantation failure. PGR knockout experiments in mouse studies demonstrated that the expression of PGR-A, but not of PGR-B, is obligatory for successful implantation and establishment of pregnancy.25 On the other hand, the overexpression of PGR-A results in uterine enlargement and endometrial hyperplasia.8,10,18 Thus, the ratio of PGR-A-to-PGR-B appears critical for the normal response of endometrium to progesterone.26
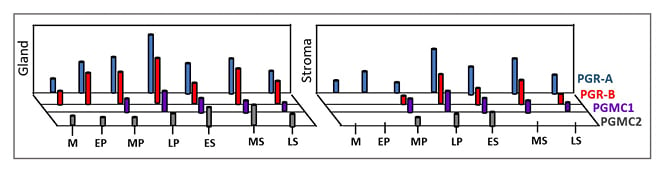
Figure 4: Expression pattern of classical progesterone receptor-A and progesterone receptor-B and membrane receptors progesterone receptor membrane component 1 and progesterone receptor membrane component 2 in glandular (shown as “Gland”) and stromal compartment (shown as “Stroma”) of human endometrium in a typical menstrual cycle.
Marked expression of PGR-A and PGRR-B in the LP and at MS is notable. PGRMC1 and PGRMC2 show subtle differential expression patterns during the menstrual cycle.
Adapted from Reis FM et al.8
EP: early proliferative phase; ES: early secretory phase; LP: late proliferative phase; LS: late secretory phase; M: menstrual phase; MP: mid-proliferative phase; MS: mid-secretory phase; PGR: progesterone receptor; PGRMC: progesterone receptor membrane component.
Among non-classical PGRs in humans, transcript levels of PGRMCs, mPRα, mPRγ, and mPRε fluctuate depending on the menstrual cycle phase. For example, levels of messenger RNAs (mRNA) for PGRMC1, mPRγ, and mPRε are upregulated during the proliferative phase and progressively decrease during the secretory phase, whereas mRNAs for mPRα and PGRMC2 are higher in the secretory phase along the increasing levels of luteal progesterone.8,16,19,24 On the other hand, the levels of mPRβ, which are relatively higher in the human endometrium than that of mPRα, do not change significantly during the menstrual cycle; however, its expression is critical on Days 10–14 of the human menstrual cycle, as revealed in patients with a history of recurrent spontaneous abortion.16,19
As seen in Figure 4, PGRMC1 and PGRMC2 show inverse expression patterns during the menstrual cycle. PGRMC1 displays regulatory action on PGRMC2. PGRMC1 is involved in cell proliferation in the endometrium during the proliferative phase of the menstrual cycle.16,19 On the other hand, PGRMC2 inhibits cell proliferation and supports endometrial differentiation during the secretory phase of the menstrual cycle.16,19,24 Thus, the reported inverse profiles of PGRMC1 and PGRMC2 in the glandular and stromal compartments of human endometrium during the menstrual cycle appears important for endometrial growth and maturation. Several lines of evidence indeed indicate that both PGRMC1 and PGRMC2, and their expression ratio in endometrial epithelial and stromal components, are critical for endometrial preparation for successful pregnancy.16,24
PHYSIOLOGICAL BASIS OF PROGESTERONE RESISTANCE
In a case report, Keller et al.27 reported on a 23-year-old female with initial complaint of infertility, who demonstrated inadequate endometrial maturation during luteal phase consistent with inadequate corpus luteum syndrome; however, she had a normal serum pattern of progesterone, oestradiol, follicle-stimulating hormone, luteinising hormone, and their cytosol-binding proteins. Exogenous progesterone did not correct the abnormality. Further investigation revealed inadequate maturation of her endometrium that caused from a markedly reduced number PGR in the target cells.27 Thus, it appeared that the condition of ‘pseudocorpus luteum insufficiency’ could have occurred due to progesterone resistance at the receptor level in the target cells of endometrium.28 Similar defect had been reported in a subgroup of females with infertility.29
Gene polymorphisms and epigenetic modifications to PGR may, theoretically, cause such progesterone resistance. Furthermore, anomalies in down-stream signalling elements, target genes, and regulator modules that are directly and indirectly linked to progesterone actions may cause functional progesterone resistance. Both types of progesterone resistance may be constitutively present in the target tissue or may be acquired by the target cells.9,10,30,31 In the following sections, the authors discuss how both types of progesterone resistance are associated with endometriosis.
PROGESTERONE RESISTANCE IN ENDOMETRIOSIS
Ectopic Tissue
There is substantial evidence to suggest that progesterone resistance is a general feature of endometriotic lesion. Table 1 lists some of the supporting studies. As mentioned above, progesterone treatment could not induce the conversion of oestradiol to oestrone in ectopic tissue.32 The enzyme 17β-HSD2, which is responsible for catalysing the reaction, is upregulated by progesterone in secretory epithelial cells of normal endometrium, but not by ectopic cells, providing the first-line evidence of progesterone resistance in endometriosis.5 In fact, PGR concentrations, for both PGR-A and PGR-B, in endometriosis tissue are generally repressed.6,33,34,44 Thus, it appears that attenuated PGR may result in altered gene transcription in ectopic tissue, even when progesterone concentration in circulation is normal.
Several large-scale gene expression studies revealed significant differences between ectopic and matching eutopic tissue. Many of the genes with differential expression were indeed progesterone target genes.37,38,42,43,45 In this regard, it is notable that there are reports of increased PGR expression in endometrioma, with a higher or unchanged PGR-B expression in endometrioma compared with normal endometrium.36,41 Interestingly, PGR expression was reportedly lower than in endometrium in primary endometriotic lesions, while in recurrent lesions there was no difference in PGR expression as compared with eutopic endometrium.46 In another study, lower PGR expression was observed in stromal cells of ectopic lesions compared with eutopic tissue, while epithelial cells of ectopic lesion showed higher PGR expression in late secretory phase.35
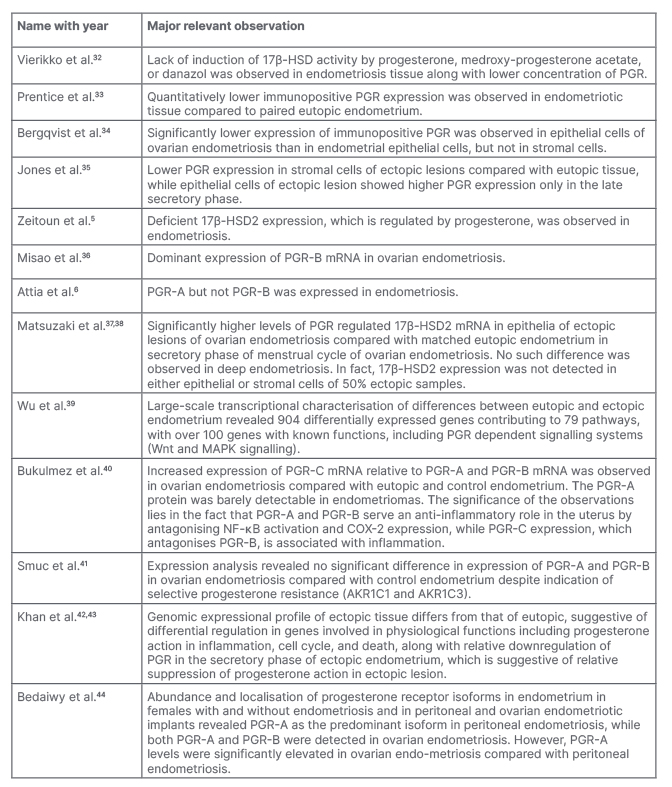
Table 1: A chronicle of reports regarding progesterone resistance in endometriotic lesion.
17β-HSD: 17-β-hydroxysteroid dehydrogenase; 17β-HSD2: 17-β-hydroxysteroid dehydrogenase Type 2; AKR1C1: aldo-keto reductase family 1 member C1; AKR1C3: aldo-keto reductase family 1 member C3; COX-2: cyclo-oxygenase-2; MAPK: mitogen-activated protein kinase; mRNA: messenger RNA; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; PGR: progesterone receptor.
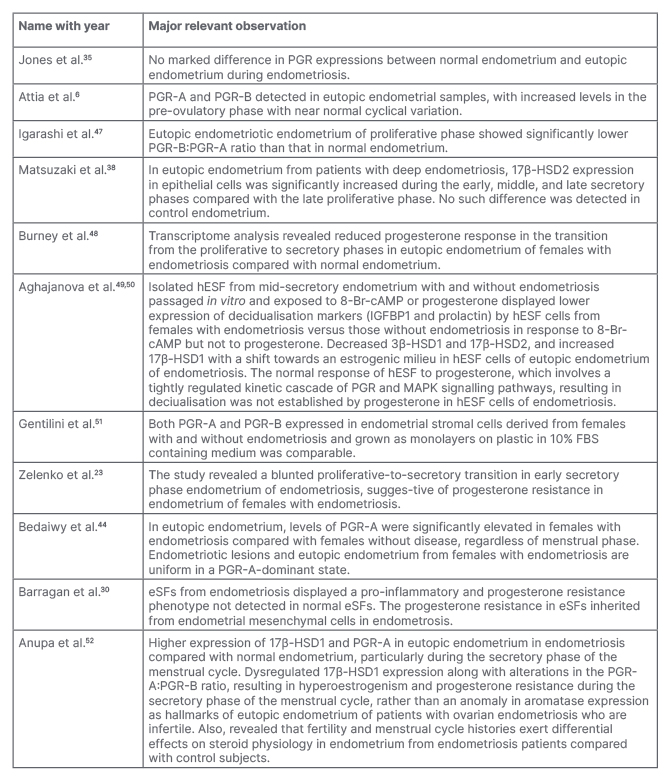
Table 2: Summary of selected reports regarding progesterone resistance in eutopic endometrium during endometriosis.
8-Br-cAMP; 8-bromoadenosine 3′,5′-cyclic monophosphate; 17β-HSD1: 17-β-hydroxysteroid dehydrogenase Type 1; 17β-HSD2: 17-β-hydroxysteroid dehydrogenase Type 2; eSF: endometrial stromal fibroblasts; FB: fibroblast; hESF: human endometrial stromal fibroblasts; IGFBP1: insulin-like growth factor-binding protein 1; MAPK: mitogen-activated protein kinase; PGR: progesterone receptor.
Eutopic Tissue
It is evident from the above discussion that ectopic tissue of females with endometriosis display suppressed PGR expression. The question whether a similar PGR suppression exists in the eutopic endometrium of females with endometriosis is rather unsettled. Table 2 provides a list of studies and the summary of results therein reflecting inconsistencies in this regard.
In a large-scale gene expression study, a higher clustering between proliferative and secretory phase samples from eutopic endometrial biopsies as compared with normal endometrium was observed, and it was associated with a substantial number of PGR target genes being affected.48 Collectively, these results were suggestive of inadequate progesterone-mediated transition of late proliferative to the early secretory phase in females with endometriosis.
Furthermore, there are studies indicating a decrease in the PGR-B:PGR-A ratio along with relatively high PGR-A expression in eutopic endometrium compared with normal endometrium.44,47,53 On the contrary, several studies failed to substantiate these findings. A cyclical variation in PGR expression in eutopic endometrium from females with endometriosis, which was comparable to normal cyclical endometriumm was reported in an early report.34 Further, several studies failed to mark any notable difference in the expression of PGR-A and PGR-B expression in normal and eutopic endometrium.6,35,51
The reported inconsistencies in results of the previous studies on endometrial progesterone receptivity might have resulted from differences in the technical details (e.g., details of tissue collection and handling) and the lack of a categorical consideration of the relative effects of fertility and menstrual histories on PGR expression and actions in the endometrium of patients with and without endometriosis.52 Moreover, the reported studies on PGR response used isolated cells maintained in a 2D culture system, which might be an inadequate model for addressing the core issue of progesterone resistance due to the fact that such isolated cells often lose their differential behaviour typically seen in the tissue.31,49,50,54
Additionally, the statistical design and clinical details in a few studies were not foolproof. The Endometriosis Phenome (and Biobanking) Harmonisation Project (EPHect) guidelines highlight the necessity of developing a consensus on the standardisation and harmonisation of phenotypic surgical and clinical data and biological sample handling methods in endometriosis research.55,56 In a recent controlled study conducted according to EPHect guidelines, lower levels of expression of aromatase and oestrogen receptor β along with higher 17β-HSD1 and PGR-A in endometrium of females with ovarian endometriosis was observed.52 Thus, dysregulated expression of 17β-HSD1 and PGR results in hyperoestrogenism and progesterone resistance during the secretory phase of the menstrual cycle, rather than an anomaly in aromatase expression, was the hallmark of eutopic endometrium from patients with ovarian endometriosis.52 It is now evident that fertility and menstrual cycle histories exert differentiating effects on endometrial physiology in females with endometriosis, vis-à-vis normal healthy endometrium.42,43,52
MECHANISMS OF PROGESTERONE RESISTANCE IN ENDOMETRIOSIS
Collectively, it appears from different lines of evidence available that progesterone resistance in endometriosis is not an ‘all-or-none’ phenomenon, and that ectopic tissue exhibits higher order of progesterone resistance than eutopic tissue in a relative scale. Suppression of PGR expression and activity in target cells may potentially take place due to interference in transcriptional, post-transcriptional, and post-translational events, and at the level of protein stability. These events can reportedly be affected in endometriosis;23,57-61 however, progesterone receptor polymorphisms are not related to susceptibility to endometriosis.62 Fundamentally, it depends on genetic background, natural history of development of the individual, and the organ and macro- to micro-environmental details.
In an interesting study, Jackson et al.63 demonstrated that PGR-A and PGR-B expression in the eutopic endometrium markedly reduced over time, between 3 and 15 months, after induction by using a model of experimental induction of endometriosis and implanting endometrial tissue into the peritoneal environment of a baboon.63 In connection to this, McKinnon et al.10 presented an elegant model explaining how constant exposure to the inflammatory ecology in peritoneal environment may result in suppression of PGR expression and activity. It now appears that human endometrial fibroblasts display PGR resistance and an inflammatory phenotype, possibly due to epigenomic modifications in the endometrium during endometriosis.10,23,30,31,40,61 Although PGR gene expressions and PGR actions are significantly dysregulated in both eutopic and ectopic tissues compared with control endometrium, the underlying epigenetic mechanisms for PGR suppression in the eutopic tissue are different from ectopic tissue.57,64
IMPLICATIONS OF PROGESTERONE RESISTANCE IN ENDOMETRIOSIS
Progesterone resistance in endometriosis has been implicated in four clinically challenging trade-offs: pain, infertility, inflammatory disorders, and neoplasm; these impair the quality of life of the patients.65 Nearly 10% of females of reproductive age have endometriosis, with more than 70% of them affected by chronic pelvic pain.65 Endometriosis is also a well-acknowledged cause of infertility, which is seen in 50% of patients with endometriosis with normal ovulation and normo‐spermic partners. Severe endometriosis is associated with poor embryo implantation rates and pregnancy rates in women undergoing in vitro fertilisation treatment.65 Females with endometriosis often have several inflammation‐linked and other comorbidities like uterine fibroid, adenomyosis, pelvic inflammatory disorder, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, fibromyalgia and cardiovascular diseases.65 Endometriosis is generally considered benign; however, unmanaged endometriosis with atypia may result in ovarian and extra-ovarian cancers.66
Based on generally known progesterone actions in target tissues as discussed above and shown in Figure 5, it appears that resistance of progesterone action may cause the above-mentioned endometriosis-associated conditions in patients.21,67 Yet, progestin treatment is met with failure in a large number of patients.7,8 Is it possible to overcome this resistance with the help of combinatorial therapies? For example, can the addition of a DNA methyltransferase inhibitor to reverse epigenetically suppressed PGR expression be helpful? The epigenetic modifications of PGR and progesterone target genes in eutopic and ectopic tissues may, however, be markedly different.57,64
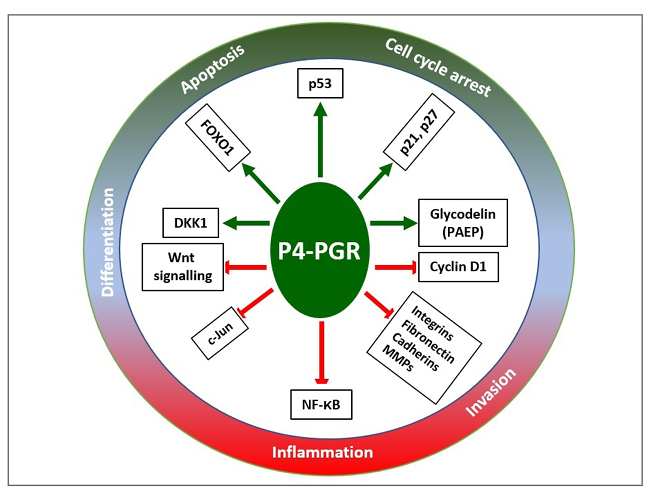
Figure 5: Progesterone mediated networks of regulatory pathways that promote (shown as the green arrow) cell cycle arrest, apoptosis, and differentiation, and inhibit (shown as the red blocked arrow) inflammation and invasion.
Adapted from Yang S et al.67
DKK1: Dickkopf-related protein 1; FOXO1: Forkhead box protein O1; MMP: matrix metalloproteinase; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; PAEP: human placental protein-14; PGR: progesterone receptor.
In primary endometriotic lesions, PGR expression is lower than in endometrium, generally having better prognosis with progesterone treatment. Meanwhile, there was no difference in PGR expression as compared with eutopic endometrium in recurrent lesions, generally having poor prognosis with progesterone treatment.46
It is evident from the above-discussion that PGR (both PGR-A and PGR-B) expression status is not sufficient to understand the nature of progesterone resistance. Can there be a set of novel and more useful functional parameters? How is PGR expression and response in target cells from females with endometriosis affected by other linked molecular mechanisms?11,21,26,68,69 Several such unmet questions point to the fact that there is an urgent need for a better understanding of the nuanced characteristics of progesterone resistance in ectopic and eutopic tissue in endometriosis, to innovate better management and treatment of progesterone resistance in the future.
CONCLUSION
It has been more than half a century since the administration of progestins has become a part of the normalised procedure to treat endometriosis. Nevertheless, its success rate is limited as it fails with time and some patients do not respond to this therapy as expected. As the authors have discussed, such progesterone resistance occurs due to supressed PGR expression, dysregulated downstream PGR actions, or both. In any case, estimated upscaling of dosage schedule or by small modifications in the molecular design of the drug cannot help in circumventing these issues. Success with available combinatorial approach is also not very promising. There is an urgent necessity for deeper and better understanding of the cellular and molecular issues related to progesterone resistance in endometriosis so that novel approaches may be innovated to restore the various homeostatic mechanisms disrupted by progesterone resistance in ectopic and eutopic tissues in endometriosis.








