Abstract
Premature ovarian insufficiency is waning of ovarian function before the age of 40 years. This hypoestrogenic state is characterised by menstrual irregularities and loss of fertility in the patient. This review narrates evaluation, consequences, and management of this complex entity. Truncation in ovarian physiology at such an early age renders the patient prone for various short- and long-term health consequences which negatively affect physical and psychological well-being of the patients. Therefore, this review emphasises that timely initiation of hormonal therapy is mandatory to mitigate the distressing menopausal and/or other hypoestrogenic symptoms to improve the quality of life of such patients. Although much has been said about premature ovarian insufficiency, many aspects of this condition still need to be explored in order to identify this population subgroup before happening of the catastrophic event and to formulate strategies and interventions to delay the premature cessation of ovarian functions.
INTRODUCTION
Premature ovarian insufficiency (POI) is a hypergonadotropic hypogonadism condition which is characterised by impairment of ovarian function on a continuum before the age of 40 years. This age limit of 40 years has been taken as the cut-off age for POI as this age is approximately two standard deviations below the natural age of menopause. However, in contrast to menopause, complete cessation of follicular function does not occur in POI and intermittent ovulation can still occur. Therefore, the term ‘premature ovarian insufficiency’ is more accepted and scientifically correct than ‘ovarian failure’. The incidence of POI is 1.0% by the age of 40 years, 0.1% by 30 years, and 1:10,000 by 20 years of age.1
POI is either spontaneous or iatrogenic in nature (surgery/radiotherapy/chemotherapy). Patients with ovarian insufficiency present either with complaint of menstrual irregularities or with hypoestrogenic symptoms. POI is the cause in 10–28% of women with primary amenorrhea and in 4–18% of women with secondary amenorrhea.2,3 Psychological impact of POI is much more than its physical counterpart and needs to be addressed while managing such patients.
Although extensive literature is available about this entity, this review comprehensively encompasses the concept of POI along with its cause, consequences, and available management options with the goal of providing relevant and current information to the healthcare provider of such patients.
METHOD
Literature was searched using the keywords ‘premature ovarian insufficiency’, ‘premature ovarian failure’, ‘premature menopause’, and other words related to the current review. The authors did not conduct an extensive search in order to find all the papers of interest acknowledging the availability of the vast literature on the topic. Studies were selected based on their relevance to the narration in the review.
PATHOPHYSIOLOGY
The number of primordial follicle peaks at 6–7 million by 20 weeks of gestation in a human female fetus. Thereafter, the follicles rapidly undergo atresia, leaving 1–2 million oocytes at birth and only 400–500 of these are ovulated before physiological menopause occurs.4 This follicular atresia is regulated by interplay of various molecules which either stimulate the atretic process (androgens, TNF-alpha, and Fas ligand) or diminish it (oestrogen, gonadotropins, and nitric oxide). Thus, attrition of follicles in POI can occur by any of the three mechanisms: a smaller number of follicles from the beginning, faster atretic rate, or impaired recruitment of the follicles.5
AETIOLOGY
The various causes of POI are summarised in Figure 1. Despite research carried out over decades to identify the cause of POI, more than half of the cases still remain idiopathic. Around 20–25% of cases have a genetic cause, out of which approximately 9% of cases are due to aberrations in the X chromosome.6 Turner syndrome is the most common chromosomal aberration abnormality in patients with POI. These patients present with primary amenorrhea and lack of breast development, while Turner mosaic patients may menstruate spontaneously. Amongst the other genetic causes, fragile X premutation is the most well studied and occurs due to change in FMR1 (Xq27.3). In this premutation, the number of CGG trinucleotide repeats increases and ranges from 55 to 200.7 Affected individuals are at increased risk of POI, from 3% in sporadic cases to 15% if a family history of POI is present.1 Any deletion or rearrangement near X inactivation centre (located at Xq13) leads to mild features in the patients which are similar to patients with Turner syndrome. Another gene located on the short arm of X chromosome which can lead to POI is BMP15.8 The protein it produces plays an important role in the maturation of oocytes. Some autosomal genes have also been identified, the mutations of which lead to decreased ovarian activity (FSHR/ LHCGR).9
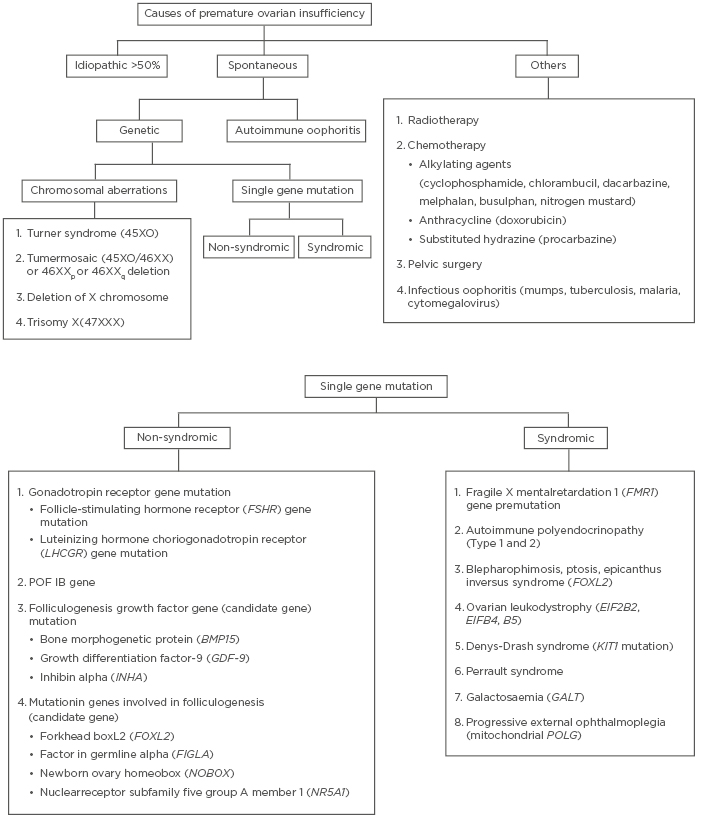
Figure 1: Summary of causes of premature ovarian insufficiency.
Various syndromes associated with POI are also enumerated in Figure 1. If damage occurs during early gonadal differentiation, it leads to the most severe form of gonadal dysgenesis. For example, in Denys Drash Syndrome, which is caused by WT1 mutation, and adrenal failure caused by SF1 mutation.5 Another important cause of POI is ovarian autoimmunity. It is diagnosed when anti-ovarian antibodies are present along with histological evidence of lymphocytic oophoritis and presence of other autoimmune disorders.10 Steroid cell antibodies (SCA) target the enzyme 17-hydroxylase and cytochrome p450 side-chain cleavage enzyme and destroy steroid-producing cells in the adrenal cortex, testis (Leydig cells), ovaries (theca cells), and placenta (syncytiotrophoblast) leading to autoimmune poly-endocrine syndrome type 1 and 2, Addison’s disease, and POI. SCA are detected in 60–87% of patients with POI-associated adrenal involvement.11 However, the major limitation in diagnosing autoimmune involvement is the high false-positive rate and poor specificity of these antibodies.12
Infectious oophoritis (viral/bacterial) has been considered as one of the causes of POI.13 Iatrogenic causes include surgical oophorectomy/radiotherapy/chemotherapy. Complete cessation of ovarian activity occurs at cumulative radiotherapy dose of 20 Gy in women <40 years and at 5 Gy in older women.14 Chemotherapy with alkylating agents, anthracycline, and substituted hydrazine increases the risk of POI by a factor of 9, more so in teenagers and young adults.15
CLINICAL ASSESSMENT
Women with POI should be asked about personal, menstrual, medical, and family history in detail. The history that needs to be asked in the questionnaire and examination points are described in Box 1. Positive family history is present in up to 30% of POI cases; therefore, detailed family history of POI should be sought.16 These patients should be asked about clinical symptoms which can be consequent to either POI alone or to associated autoimmune/genetic syndromes. Patients with POI experience menopausal symptoms such as vasomotor symptoms (commonest),17 altered mood, tiredness, vaginal dryness, dyspareunia, urinary frequency, incontinence, and reduced libido. Around 12–14% of patients do not experience any menopausal symptoms,18 especially patients in whom POI occurred before menarche because these symptoms are largely due to oestrogen withdrawal, and not deficiency.
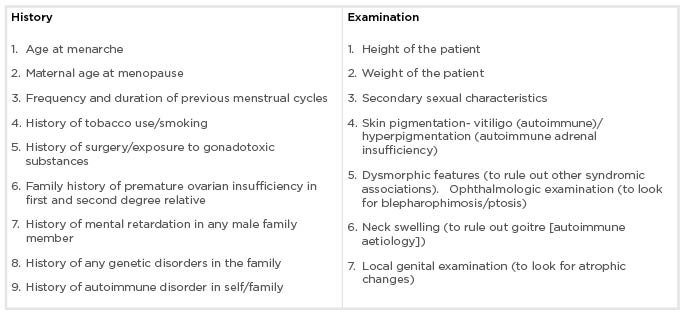
Box 1: Clinical history and examination of patients with premature ovarian insufficiency.
INVESTIGATIONS
Diagnostic work-up of such patients should include confirmation of the diagnosis and the ruling out of other comorbid conditions. Although different levels of follicle-stimulating hormone (FSH) have been taken as cut-off value, persistent rise of serum FSH level >30 IU/L on two different occasions, at least 4–6 weeks apart, confirms the diagnosis of POI in the setting of menstrual irregularities.19-23 Other common causes of amenorrhea should be excluded, such as pregnancy, hyperprolactinemia, chronic medical illness, and polycystic ovarian syndrome. Investigations that should be performed in all the cases are summarised in Box 2. A progesterone withdrawal test should not be completed as it gives a false sense of assurance thus delaying the diagnosis.24
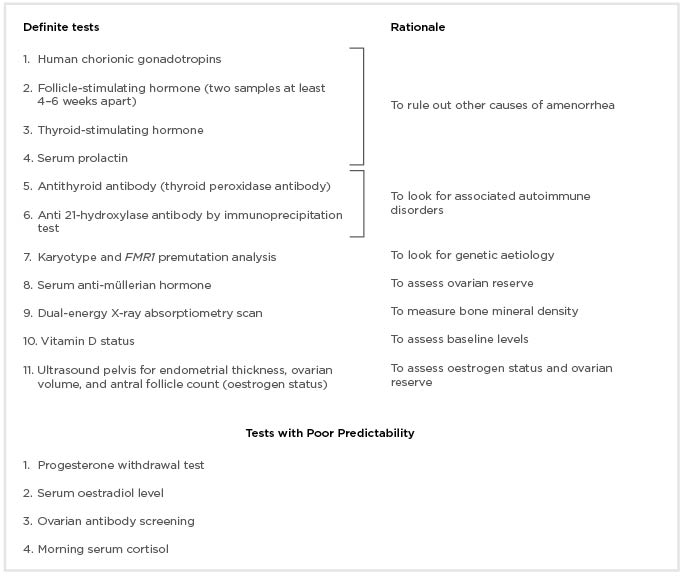
Box 2: Investigations to be done in all cases of premature ovarian insufficiency.
Once the diagnosis of POI is confirmed, one should look for the cause of this condition. Chromosomal analysis, fragile X premutation testing, screening for thyroid antibody, and the measuring of 21- hydroxylase antibody levels can be done to confirm aetiology in patients with POI, wherever needed.25-27 Autosomal gene mutation testing and anti-ovarian antibody testing is not routinely recommended. Ovarian biopsy is the gold standard test to diagnose ovarian autoimmune disorder. However, being an invasive test, it is not performed routinely. A bone density scan (DEXA scan) should be done to measure baseline bone mineral density (BMD) and if the bone density is in the normal range and the patient is compliant on hormone replacement therapy (HRT), this test can be repeated every 2–3 years.18
LONG–TERM CONSEQUENCES
Premature withdrawal of oestrogen leads to premature ageing of various organ systems which are a primary target of oestrogen action and this may manifest as various diseases/disorders in the body. These effects are summarised above.
Cardiovascular System
Cardiovascular diseases are the main culprit behind shortened life expectancy of patients with untreated POI. A significant association was found between cardiovascular disease and early menopause in a meta-analysis.28 Rocca et al.29 showed that mortality was significantly increased in women who had a bilateral oophorectomy before the age of 45 years compared to those women who did not have an oophorectomy. In another study by Gordon et al.,30 postmenopausal women in their fourth decade were found to have increased incidence of cardiovascular disease compared to premenopausal age-matched controls.
Bone Health
Lack of oestrogen increases bone remodelling, but bone resorption exceeds bone formation. The net effect is bone loss and in addition to that, slow mineralisation of new bone leads to low BMD. Almost 50% of affected women have significantly reduced BMD within 18 months of diagnosis and 2/3 have low BMD levels which put them at a high risk of hip fractures.31,32 After 2–9 years of the diagnosis, there is a reduction of 2–3% in BMD at spine and hip.18
Effect on Cognition
Women with POI are at increased risk of cognitive impairment. Increased risk of cognitive impairment or dementia has been seen in women who undergo oophorectomy (unilateral/bilateral) before the onset of menopause.33 Bove et al.34 reported that premature surgical menopause is associated with faster decline in global cognition and memory. It was also studied that a HRT duration of at least 10 years (if started within 5 years of perimenopausal period) had protective effect on global cognition.
Genital Organ Dysfunction
Women with POI might have poor sexual performance owing to pain and vaginal dryness because of the impact of a lack of oestrogen. Androgen deficiency is the cause of low libido, poor genital arousal, and orgasm, as well as of blunted motivation and diminished wellbeing.35,36 The loss of follicular function will result in infertility.
Psychological Effect
Sudden diagnosis of loss of fertility and menstruation can be unexpected and women often express anxiety, depression, or anger that is usually underestimated by the clinicians. It can be associated with increased lifetime risk of major depression and anxiety.37,38 Women with POI have lower perceived social support and self esteem, which in turn affects their quality of life.39,40
MANAGEMENT
POI should ideally be managed by a multidisciplinary team comprising a gynaecologist, an endocrinologist, and a psychologist. The treatment of POI has the following components:
- HRT.
- Fertility regulation.
- Psychological support.
Hormone Replacement Therapy
Replacement of oestrogen is the first-line treatment of POI with the aim of relieving menopausal symptoms and improving cardiovascular, sexual, and bone health. It can induce secondary sexual characteristics in adolescents with POI. If breast development is incomplete or absent, oestrogen therapy should be initiated in low doses and should be slowly increased before starting progesterone to avoid tubular breast formation.23
Benefits of Hormone Replacement Therapy
HRT improves lipid profile by increasing serum high-density lipoprotein and decreasing total cholesterol and low-density lipoprotein. Overall, there is a 24% reduction in the risk of coronary artery disease after starting HRT.41 It improves BMD and thus significantly reduces risk of fracture.42 It also reverses the urogenital atrophy caused by oestrogen deprivation as well as relieves vasomotor symptoms. Thus, it improves the overall quality of life of the patient.
Routes of Administration
Oestrogen can be given orally or by a transdermal route. The transdermal route is preferred as the first pass effect by the liver is eliminated, thus lowering the risk of venous thromboembolism (VTE).43 A study by Langrish et al.44 showed better cardiovascular health outcomes amongst women with POI who received transdermal oestradiol and norethisterone. Oestrogen can be given as either 1–2 mg oral 17-β-oestradiol daily, 100 μg transdermal oestradiol, or 0.625–1.250 mg conjugated equine oestrogen. Progesterone can be administered by oral/transdermal/uterine route to prevent endometrial hyperplasia that can result from unopposed action of oestrogen in patients with an intact uterus (continuously/sequentially). Women can choose to have a levonorgestrel intrauterine device if they want effective contraception also. HRT has to be started soon after the diagnosis of POI and should be continued until the age of natural menopause.
Loss of ovarian activity can reduce total androgen production by 50%, which can affect sexual health. Therefore, if needed, androgens can be replaced in the form of testosterone cream/gel/implant/patch.
Combined Oral Contraception versus Hormone Replacement Therapy
There are no large, well controlled trials to compare combined oral contraception (COC) and HRT for the treatment of POI. As COC is much more potent than HRT, such a strong action is not required for the treatment of POI. However, it is easy to take and is associated with less stigma. There are only a few trials comparing the effect of HRT and COC, but these studies are grossly underpowered to draw any meaningful conclusion. Langrish et al.44 compared HRT (transdermal oestradiol 100–150 μg/day and vaginal progesterone 400 mg/day) versus COC (30 μg ethinyl oestradiol with 1.5 mg norethisterone) in a randomised, controlled, crossover trial and found that treatment with HRT resulted in lower mean 24 hour systolic and diastolic blood pressure over 1 year when compared to COC. Women who were on HRT had increased BMD and bone formation markers when compared to women who were given COC.45 Cartwright et al.46 also found that HRT administration (2 mg of 17-β-oestradiol and 75 μg levonorgestrel) significantly increased lumbar spine BMD at 2 years compared to COC administration (30 μg ethinyl oestradiol with 150 μg levonorgestrel) in patients with POI. Treatment with oestrogen and progesterone can be given either as either HRT or as COC until the results of larger randomised trials are available.
Side Effects of Hormone Replacement Therapy
In menopausal women, oral oestrogens increase the risk of VTE, the risk being the highest in the first year of its use. However, whether this data from older women can be extrapolated to young women still remains unanswered. Nevertheless, in order to reduce the risk of VTE, a transdermal route is preferred over an oral route.43,47
There is insufficient data to evaluate the association between oestrogen therapy given to women with POI and risk of developing breast cancer. A large population-based study including data from Danish Cancer Registry shows that the risk of breast cancer was increased in women on HRT aged ≥50 years, whereas the risk was not increased in women <50 years of age.48 To prevent the risk of endometrial hyperplasia and carcinoma, progesterone should always be included in HRT to avoid unopposed action of oestrogens.
Other Measures for Bone Health
Bisphosphonates work by inhibiting the activity of osteoclasts and thus reduce bone resorption. However, it is not preferred in women of reproductive age group who are desirous of future pregnancy. Moreover, it is associated with osteonecrosis of the jaw and subtrochanteric fracture of the femur if taken for a long time. Weight bearing exercises should be done along with avoidance of smoking tobacco and drinking alcohol. Vitamin D status should also be measured and replenished if it is <30 IU/L. Calcium should also be supplemented in the dose of 800–1,000 mg/ day.18
Fertility Regulation
There are various treatment options to improve fertility in these women. In 5–10% of these women, there is spontaneous resumption of ovulation which can lead to pregnancy.49 Thus, women not willing for future pregnancy should be offered a definite contraception.
Management of Infertility
The ‘wait and see’ approach is not appropriate for infertility treatment in patients with POI because ovulation is intermittent and unpredictable in these patients. However, spontaneous pregnancy with idiopathic POI is not associated with increased risk of miscarriage and obstetric complications.18 A mixed retrospective and prospective study showed that 24% of women with idiopathic POI resumed ovarian function and the majority of the patients had resumption within 1 year of diagnosis.50
Pre-treatment with oestrogen suppresses FSH levels and allows restoration of FSH receptors in remaining follicles. Pre-treatment with oestrogen followed by exogenous gonadotropin stimulation resulted in ovulation in 32% of cases and pregnancy in 16% cases.51 In vitro fertilisation with donor oocyte has the highest chance of pregnancy. In this, endometrium can be prepared for implantation by escalating the dose of oestradiol valerate along with natural progesterone. Pregnancy with donor oocyte is associated with complications such as hypertensive disorder, growth restriction, and preterm delivery.52 It has been postulated that immunologic intolerance between mother and fetus is responsible for the complications. Dehydroepiandrosterone promotes activation of oocytes and inhibits their atresia. Higher pregnancy rates with dehydroepiandrosterone supplementation has been noted in patients who have diminished ovarian function.53
Other new options are ovarian cortex transplantation and transplantation of an entire ovary.54,55 Auto-transplant in cancer patients may be associated with the risk of dissemination. Thus, some authors have tried transplantation in monozygotic twins discordant for ovarian failure.54 In vitro maturation of oocytes derived from preantral follicles which are spared in POI can be used as one of the treatment options.1
Ovarian Preservation56,57
To avoid follicular damage in young women requiring chemotherapy or radiotherapy for cancer, the following measures can be taken:
- Gonadal shielding
- Ovarian transposition
In this technique, ovaries are moved out of radiation field by cutting the utero-ovarian ligaments and mobilising them in patients undergoing radiotherapy for cancer management. The procedure has certain complications which include chronic pelvic pain and post-operative adhesions. Sometimes, the procedure itself may cause premature ovarian failure. Ovarian transpositioning prevents the ovaries from radiation induced injury only. Therefore, if a patient requires both chemotherapy and radiotherapy, this should be avoided.
- Ovarian suppression by GnRH analogues
GnRH agonism suppresses FSH and luteinising hormone levels and protects the ovarian follicles from destruction by the chemotherapy by suspending ovarian function temporarily. Therefore, women in the reproductive age group should receive a GnRH agonist along with chemotherapy.
- Cryopreservation of oocyte/embryo/ovarian tissue
(i) Embryo/oocyte cryopreservation:
Both embryo/oocyte cryopreservation requires controlled ovarian hyperstimulation and subsequent oocyte retrieval. This procedure demands delay of a minimum of 14 days before starting chemotherapy. To preserve embryos, in vitro fertilisation with either male partner or donor sperm is needed. Embryo cryopreservation has a good success rate in terms of cumulative pregnancy rates. Oocyte cryopreservation is more useful when a patient does not have partner. Oocytes are retrieved after ovarian stimulation and stored for future purposes. The cryopreservation of embryos and oocytes has a disadvantage that only a limited number can be stored at one time, thus limiting the number of attempts for future pregnancies.
(ii) Ovarian tissue cryopreservation:
This method requires ovarian tissue harvesting by laparoscopic procedure and storage of the tissue for future purposes. The advantage of this technique is that it does not demand hormonal stimulation of the ovary and there is no significant delay in initiation of chemotherapy.
Contraception
Because 5–10% of women with POI can have spontaneous resumption of ovulation, contraception should be provided to women not desirous of future fertility. Although combined oral contraceptive pills are one of the most commonly followed methods of contraception, they may fail to suppress the high FSH levels seen in POI and thus can result in pregnancy. There have been anecdotal reports of women with POI having conceived after taking oral contraceptive pills.58 Barrier contraception or intrauterine devices are better options for these women.20
Social and Psychological Support
Many women with POI are not satisfied with the amount of information shared with them by their clinicians.59 Family should be involved at the time of breaking bad news. Psychological support should be given in the form of counselling to the patients and appropriate treatment to those suffering from anxiety or depression. The diagnosis of POI, and its effects, can alter their quality of life. Large support groups for such women are available, such as the Daisy Network Premature Menopause Support Group and the International Premature Ovarian Failure Association. Considering the sensitive nature of diagnoses, as well as cultural significance, patients should be informed of the diagnosis in a gentle manner, ensuring family support is available. Parents of adolescents with POI should also be counselled regarding the consequences and must be primed for providing emotional support to their daughters.23
FUTURE RESEARCH
An international research consortium and disease registry should be formed to provide clinical data regarding pathogenesis and management of POI.60 Transplantation of ovarian cortex and activation of dormant follicles using a PTEN inhibitor in the re-implanted tissue needs further research. Large, randomised, controlled trials are required to compare various HRT options for their safety and efficacy. Until then, a multidisciplinary approach is the need of the era to identify such patients and provide them with optimum care with the utmost psychological support.







