Abstract
The use of assisted reproduction techniques has been associated with obstetric complications. An understanding about which methods and treatment protocols produce better outcomes would provide greater opportunities for a successful pregnancy. The aim of this literature review was to identify whether frozen embryo transfer (FET) leads to a greater incidence of pregnancy-induced hypertension (PIH) compared to fresh embryo transfer. Fifteen studies were identified and subsequently reviewed. Eleven studies suggested FET increased the incidence of PIH–gestational hypertension and pre-eclampsia. The evidence suggests a correlation between FET and PIH. Exploration into why this is the case should be the focus of future studies. Implications for clinical practice involve extensive preconception counselling and potentially advising prophylactic low-dose aspirin with the aim of lower the incidence of PIH.
INTRODUCTION
Subfertility affects 1/6–7 couples worldwide.1 Assisted reproduction techniques such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) are treatment options for these couples. Hypertension-related disorders affect 8–10% of pregnancies in the UK.2 It can cause fatal consequences, such as premature labour and intrauterine growth restriction, and maternal consequences, e.g., liver damage and stroke.3 Using fresh or frozen embryos are both options for IVF/ICSI under the National Institute for Health and Care Excellence (NICE) guidelines.1 The Human Fertilisation and Embryology Authority (HFEA)4 reports approximately one-third of IVF/ICSI cycles result in frozen embryos. It would be beneficial to better understand the effect of frozen embryo transfer (FET) related to obstetric outcomes so that optimal care can be provided. A literature review was performed to assess the current evidence to determine whether FET leads to a greater rate of pregnancy-induced hypertension (PIH), e.g., pre-eclampsia (PE) and gestational hypertension (GH). The objective of this review was to identify whether FET use in IVF/ICSI leads to a greater incidence of PIH, specifically, PE and GH, compared to when fresh embryo transfer (ET) is used.
METHOD
Objective
To identify whether FET use in IVF/ICSI leads to a greater incidence of PIH, specifically PE and GH, compared to when fresh ET is used.
Outcomes
The primary outcome was the development of PIH, to include GH and PE.
A Medline search was performed from inception until April 2020. The following search terms were used: “assisted reproductive techniques”, “assisted reproductive treatment”, “assisted reproductive technology”, “in vitro fertilisation”, “IVF-ET”, “embryo transfer”, “intracytoplasmic sperm injections”, “oocyte donation”, and “sperm donation” alongside “pre-eclampsia”, “pregnancy induced hypertension”, “gestational hypertension”, and “eclampsia”, alongside “cryopreservation”, “vitrification”, “fresh embryo”, “frozen embryo”, “frozen blastocyst”, and “blastocyst vitrification”.
Sixty articles were found and screened by reading the title and abstracts. Studies that compared the use of FET and fresh ET and the development of PIH were selected. Inclusion criteria for the selected studies were English-language papers, human studies, randomised controlled trials (RCT), cohort studies, case-control studies, retrospective, and prospective studies. Exclusion criteria were systemic reviews/meta-analysis, letters to the editor, and case reports/abstracts proceedings (grey literature). Nineteen articles were included in the final analysis. The screening process is summarised in Figure 1.
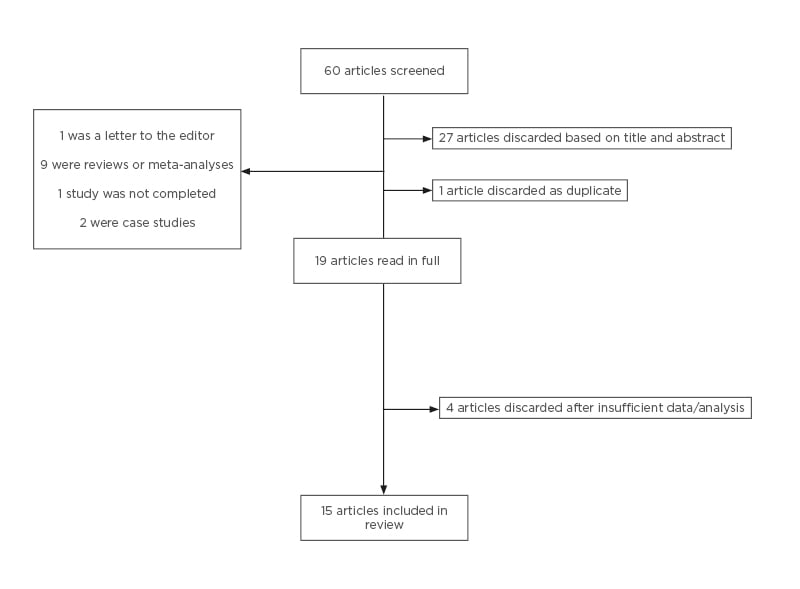
Figure 1: Screening process of articles after keyword search.
RESULTS
Following the search, 19 articles were retrieved in full, and four were excluded because they did not have the appropriate data or analysis comparing FET and fresh ET (Figure 1). Fifteen articles fulfilled the inclusion criteria. Tables 1 and 2 outline the study type, main results, and comments about the included articles. Table 1 includes articles relating to PE and GH as separate outcomes.
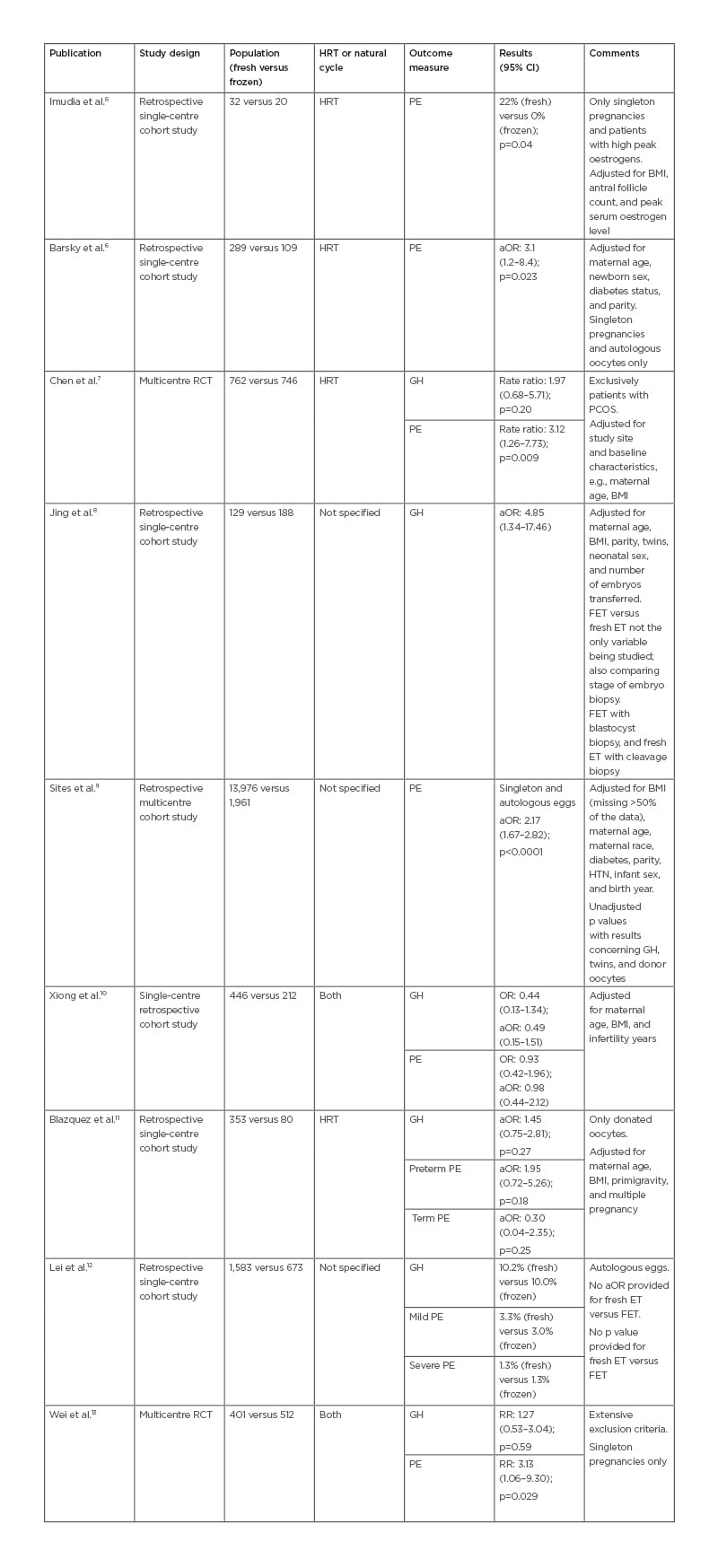
Table 1: Study characteristics and incidence of pre-eclampsia and gestational hypertension.
aOR: adjusted odds ratio; ART: assisted reproduction technique; CI: confidence interval; ET: embryo transfer; FET: frozen embryo transfer; GH: gestational hypertension: HTN: hypertension; HRT: hormone replacement therapy; OR: odds ratio; PCOS: polycystic ovarian syndrome; PE: pre-eclampsia; RCT: randomised controlled trial; RR: relative risk.
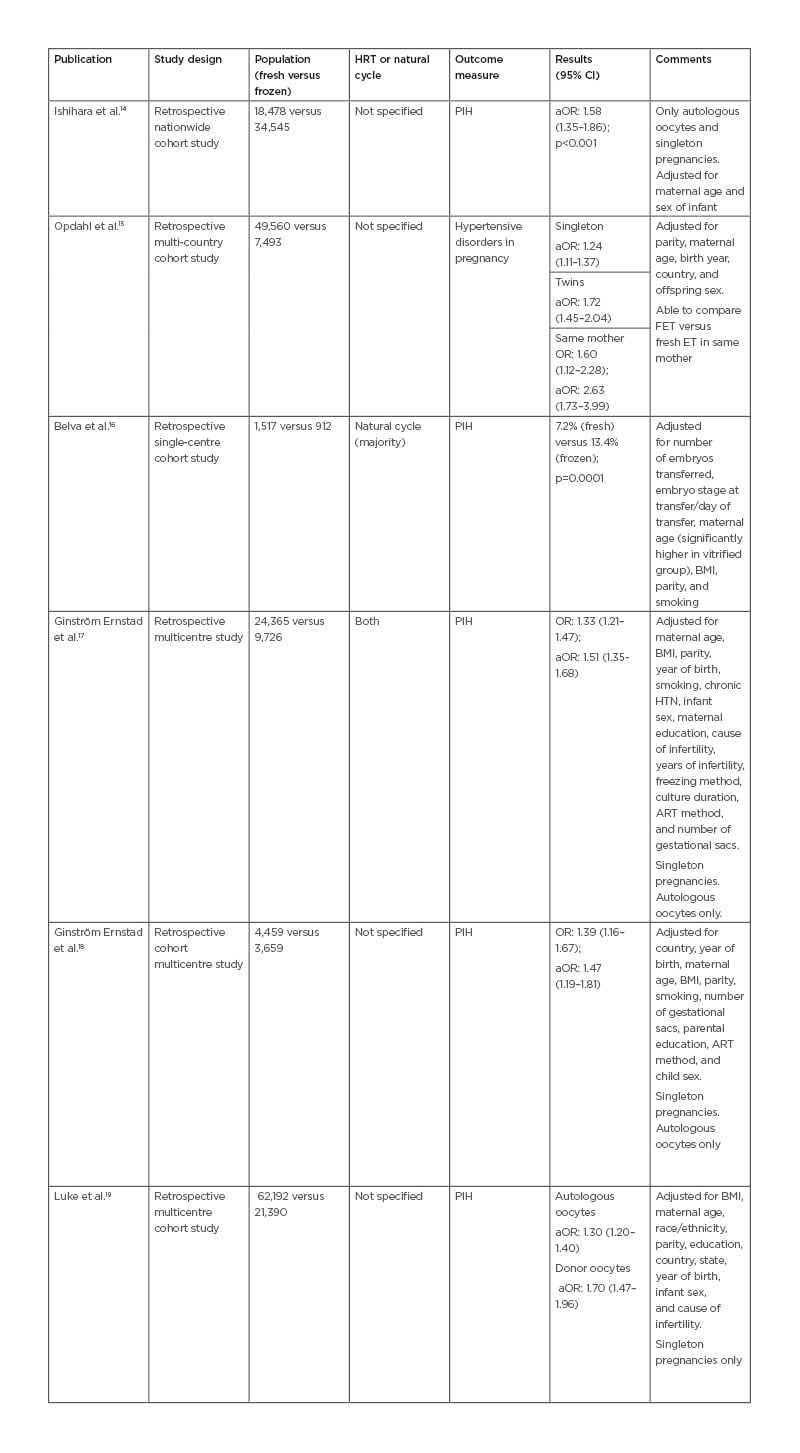
Table 2: Study characteristics and incidence of pregnancy-induced hypertension and hypertensive disorders in pregnancy.
aOR: adjusted odds ratio; ART: assisted reproduction technique; CI: confidence interval; ET: embryo transfer; FET: frozen embryo transfer; HTN: hypertension; HRT: hormone replacement therapy; OR: odds ratio; PIH: pregnancy-induced hypertension; RR: relative risk.
Table 2 includes articles relating to PIH and hypertensive disorders in pregnancy as a single outcome. PIH includes GH, PE, and eclampsia. Hypertensive disorders in pregnancy refer to conditions associated with PIH, as well as pre-existing hypertension complicating pregnancy.
Of the 15 articles reviewed, summarised in Tables 1 and 2, a majority (11/15) of papers found that FET confers a higher risk of developing PIH.6-9,13-19 Chen et al.7 and Wei et al.13 found an increased incidence of PE but not GH in the FET group. After adjusting for maternal age, BMI, parity, multiple gestations, and smoking, excluding donor oocytes, the results were consistent (Tables 1 and 2). Imudia et al.5 found that fresh ET conferred a higher risk of PE compared to FET.
DISCUSSION
The results suggest that FET increases the incidence of PIH. A variety of studies display this correlation, including RCTs. Each study focussd on different elements and populations giving validation to this claim. The main limitations of these results are the quality of the evidence presented.
Risk Factors of Pre-eclampsia
When looking at the evidence presented, the impact of confounders on the results must be considered, such as risk factors for PE, which include older age, multiple gestation, BMI, past medical history (PMH) of PE, diabetes, family history of PE, and parity.20,21
Most of the studies had information on risk factors and adjusted their results accordingly (Tables 1 and 2), finding consistent results. However, most of the studies did not adjust for PMH,5-12,14-19 which is a significant risk factor for PIH. Bartsch et al.,20 who conducted a meta-analysis of over 90 studies, found that PMH of PE increased the risk of developing PE in subsequent pregnancies by eight times. The same study found that pre-gestational diabetes almost quadrupled the risk of developing PE.20 Nonetheless, the studies that adjusted for or excluded pre-gestational diabetes still found that FET increased the risk of developing PIH.6,9,19
IVF/ICSI is a complex process with many variables that may factor into the development of PIH. This information was not always accounted for.6,9,10,14,15,17-19 Embryo biopsy for pre-implantation genetic testing and donor oocytes have been associated with significantly increased risk of PIH.22-24 However, studies excluding donor oocytes still had an increased risk of PIH during FET.6,9,14,17-19
Natural versus Hormone Replacement Therapy Cycle in Frozen Embryo Transfer
A potential factor in developing PIH during FET is the type of endometrial preparation used for the FET cycle: natural versus hormone replacement therapy (HRT). Of the 15 articles reviewed, eight commented on the method used for endometrial preparation (Tables 1 and 2). This is a confounding factor that most studies have not adjusted for (Tables 1 and 2). A specific analysis comparing HRT versus natural cycles in FET found that HRT cycles lead to an increased risk of PIH compared to natural cycles, adjusted odds ratio 1.78,17 suggesting a role for HRT in the development of PIH.
The mechanism by which HRT cycles, commonly used in FET, increase the risk of PIH is still debated. A suggested mechanism is the absence of the corpus luteum (CL), a feature of HRT cycles; subsequently, the absence of hormones produced by the CL could adversely affect the maternal cardiovascular system.25,26 von Versen-Höynck et al.26 found that HRT with no CL had a significantly higher incidence of PE compared to natural FET with one CL.
Study Type
The study type will contribute to the quality of the evidence. The majority of the papers were retrospective cohort studies (Tables 1 and 2). One of the major limitations is that information on confounding factors may not be available. Another disadvantage is that any missing information deemed important would be difficult to find and follow up. However, retrospective cohort studies have the advantage of low risk of response bias because individuals did not know what the data would be used for, and large sample sizes, which allow studies to be more representative of the general population.
The highest-quality evidence comes from RCTs. RCTs reduce the risk of selection bias because participants are randomly assigned to each group. Unfortunately, these trials were not double-blinded, and in this circumstance, may not be ethically possible. RCTs are able to control a variety of potential confounders. Both of the RCTs found an increased risk of PE when FET was used, but GH incidence was not affected.7,13
Multicentre studies have the added benefit of reduced selection bias because of the wider range of participants available, and allow conclusions to be made with regard to a larger patient base (Tables 1 and 2). However, there is a risk of performance bias because the patients are more likely to have differences in their prenatal/antenatal care and treatment. Some of the studies had a wide date period of 10+ years.15,17-19 This leads to an extensive sample size, reducing selection bias; however, it can expose the study to unknown confounders because medical practices and lifestyle can change during that time period.
A Nordic study15 found in the same mother the chance of developing PIH was increased by 2.5 times when using FET compared to fresh ET.15 This is strong evidence because many maternal confounding factors can be removed with this model, suggesting the correlation between FET and PIH is not solely due to maternal factors. However, the sample size for this analysis was small.
All four of these papers that did not see a correlation between PIH and FET are single-centre studies.5,10-12 The advantage is that there is a reduced risk of confounders associated with variation of prenatal/antenatal care and guidelines affecting the results. However, they are more likely to have smaller sample sizes.5,11 The four studies had an increased risk for Type 2 error, where a difference in treatment outcome is not seen but there is one present, because of a significantly smaller sample size in the FET versus fresh ET group. Blazquez et al.11 had a wide confidence interval, which makes it difficult to interpret where the true odds lie. The evidence provided by Lei et al.12 is limited because of a lack of statistical data, i.e., the p value.
Previous Studies
Previous reviews and meta-analyses have also found an increase of PIH in patients with FET.27-29 Maheshwari et al.27 included five studies in their meta-analysis and found FET led to a 29% increased risk of PIH (relative risk [RR]: 1.29). Similarly, Sha et al.28 reviewed seven studies and found a 44% increased risk of developing PIH when using FET (RR: 1.44) in relation to PIH development. However, these reviews also showed a lower risk of other obstetric complications, e.g., preterm delivery, placenta praevia, and placental abruption.27,28 This suggests there is a role for FET and that special considerations should be taken when comparing the options.
Clinical Implications
Clinically, these data suggest a possible use of preventative treatment, e.g., low-dose aspirin during FET in high-risk individuals. The efficacy of aspirin in preventing PE has been replicated in >70 trials and reduces the risk of developing PE by 18% (RR: 0.82).30
NICE31 recommend that patients with risk factors take aspirin daily from the 12th week of gestation. Based on the evidence presented, there may be a role for including FET as a risk factor. Compared with other risk factors, such as PMH or family history of PE, the chance of developing PE with FET may not be increased as dramatically. However, in the setting of assisted reproduction techniques, where there are often limited chances, it seems worth investigating a role for prophylaxis. Clinicians should consider their use of FET when weighing up the risk of PIH and monitor patients more closely.
CONCLUSION
This literature review comparing the effect of FET and fresh ET on development of PIH shows there is an association between FET and PIH. There was a large number of articles15 reviewed, including two RCTs, compared with previous reviews, which included only 2–7 articles and were limited to only observational studies.27-29 This review could highlight a role for use of preventative treatment during FET in high-risk individuals. It also suggests the importance of providing appropriate counselling during the FET cycle and potential referral for antenatal counselling when other risk factors are associated. This is the first review to discuss the potential use of aspirin in FET. Though there is a correlation established, future studies exploring the reasons for FET conferring a greater risk are needed. This could be due to embryo quality following the cryopreservation and thawing procedures or different intrauterine environment, e.g., absence of the CL,17,25 or ultimately, to the hormonal treatment and protocols associated with FET.15







