Abstract
Nowadays, sperm cryopreservation is strongly recommended in cases of malignancy. Moreover, the use of frozen testicular sperm in azoospermic patients prevents the need for repeated sperm retrieval and optimises scheduling between oocyte and sperm obtainment. Even though cryopreservation of human spermatozoa for assisted reproductive purposes is a widely implemented practice, none of the established freezing and vitrification techniques offer optimal cryosurvival results due to the dramatic impact of cryodamage on sperm cells.
This comprehensive review describes the most commonly used sperm cryopreservation techniques in order to establish which of them minimise sperm cryodamage and offer better survival rates.
Presently, it is not sufficiently demonstrated that sperm vitrification improves survival significantly more than freezing methods. Slow freezing offers the best survival results when compared to other freezing protocols, and owing to its technical advantages, can be considered as one of the preferred protocols to be easily implemented in assisted reproduction laboratories.
Moreover, several studies have suggested that sperm preparation prior to cryopreservation can improve thawed sample quality. However, other authors have demonstrated that freezing the fresh sample and performing semen preparation after thawing gives better results in regard to total motile sperm count and motility.
Regarding clinical results, it is well established that similar or even better reproductive outcomes are achieved using frozen testicular sperm in cases of azoospermia or anejaculation. Moreover, the use of frozen semen in cancer patients can help to achieve good fertilisation and pregnancy rates. Finally, the use of frozen sperm is not at all associated with worse post-natal development.
INTRODUCTION
The cryopreservation of human spermatozoa for assisted reproduction techniques is a clinical practice implemented worldwide since its early beginnings in the 1970s. Since then, the development of new protocols aiming to optimise the quality of thawed sperm samples has been of major interest to researchers and constantly pursued in the field of andrology. Besides this, sperm cryopreservation has become a paramount facet of ART programmes since it has allowed the establishment of donor sperm banks. Furthermore, it is, to date, the treatment of choice to preserve fertility in pubertal children and adults.
Sperm cryopreservation can be achieved through both equilibrium and non-equilibrium procedures, which are commonly referred to in the literature as sperm freezing and vitrification, respectively. In spite of efforts to implement human spermatozoa vitrification as a routine protocol in clinical practice, conventional freezing is still the most widely used protocol for clinical purposes.
Sperm freezing is used routinely to cryopreserve not only ejaculated but also non-ejaculated spermatozoa retrieved from azoospermic patients who have undergone a sperm extraction surgery.
Unfortunately, as a result of the impact of cryopreservation-associated damage to spermatozoa, the viability rates of thawed samples remain low. Cryopreservation-induced damage is due to both mechanical and osmotic stress, and although cryoprotectant compounds are necessary for sperm freezing, they are also a source of osmotic damage when their levels exceed optimum concentration.
Due to this, the optimisation of cryopreservation protocols to increase the number of viable spermatozoa in thawed samples must be a major concern for all male patients undergoing in vitro fertilisation treatments. Nevertheless, it is even more crucial when applied to patients undergoing gonadotoxic treatment for malignancies and non-malignant chronic diseases, and who are counselled to attend fertility preservation programmes: these pathologies, and their associated therapies, threaten these patients’ fertility potential, thus limiting their sample availability.
In this scenario, it is worth mentioning that sperm selection techniques, such as through swim-up and density gradients centrifugation (DGC), are essential steps to be performed prior to using a sperm sample for intrauterine insemination or in vitro fertilisation. However, the sequence in which sperm selection and cryopreservation must be performed remains controversial, since the protective role of the seminal plasma during sperm freezing must not be dismissed.
This review will provide comprehensive information regarding the state-of-the-art human spermatozoa cryopreservation protocols and compare their outcomes.
METHODS
An exhaustive literature review of the current protocols implemented for clinical purposes and the reproductive outcomes derived has been performed. Topic-relevant scientific papers published in PubMed are included. Keywords used for the bibliographic search were “sperm freezing”, “sperm vitrification”, “sperm preparation”, “total motile sperm count”, and “sperm viability”.
Sperm Cryopreservation Protocols
Equilibrium Procedures: Sperm Freezing
The impairment of sperm cell parameters during cryopreservation is partly due to physical changes related to ice-crystal formation that in turn result in mechanical damage to spermatozoa. In order to prevent intracellular ice-crystal formation, measures to be considered are either controlling freezing rates or promoting cellular dehydration by using cryoprotectants. However, cryopreservation-associated damage is also a response to osmotic stress caused by the addition of cryoprotectants. Cryoprotectant agents are necessary compounds for sperm cryopreservation, particularly for the freezing stage, since they protect against ice-crystal formation. Cryoprotectants can be divided in two groups, dependent on their capability to pass through the sperm cell membrane: permeable cryoprotectants are compounds of low molecular weight capable of infiltrating the cell membrane, whereas non-permeable cryoprotectants are compounds that cannot penetrate the membrane and so force dehydration by means of increasing the osmotic concentration of the extracellular media. With regard to sperm freezing, the most widely used cryoprotectants are egg yolk and glycerol. Lipoproteins present in egg yolk help minimise damage to the sperm cell membrane during cryopreservation, whereas glycerol, a permeable cryoprotectant, balances the solute concentration of intra and extracellular media (Table 1A). Nevertheless, the addition of glycerol has to be done progressively since it spreads into the sperm cell cytoplasm more slowly than water, and so changes in cell volume must be controlled.
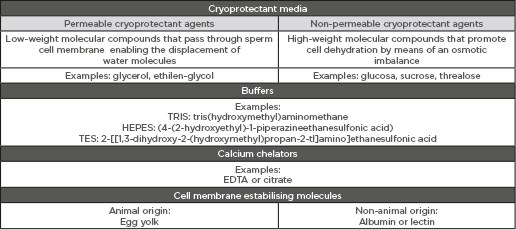
Table 1A.
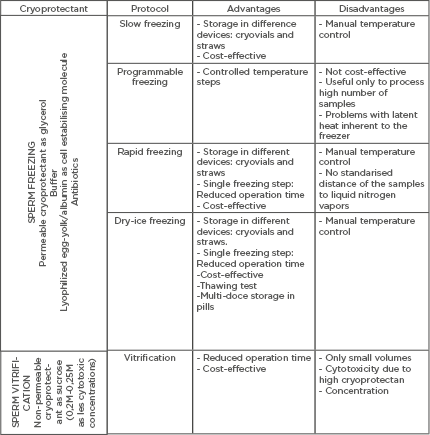
Table 1B.
A) Summary of composition of cryopreservation media. B) Most commonly-used cryoprotectant media used for sperm freezing and sperm vitrification. General description of the advantages and disadvantages of each cryopreservation protocol.
Slow Freezing
Slow freezing is one of the most commonly used protocols for human sperm cryopreservation. The procedure can last from 30 minutes to 1 hour and is an equilibrium technique characterised by the maintenance of osmotic balance during the temperature decrease. This protocol requires the use of cryoprotectants, mainly derived from egg yolk. Firstly, the sperm sample is mixed gradually, volume to volume, with cryoprotectant before being homogenised. Slow freezing consists of two freezing steps so that the temperature decrease occurs progressively. Firstly, the sample is mixed with cryoprotectant agents and then incubated at 4 °C for 20 minutes. Following this, the temperature decreases from 4 °C to -80 °C, meaning the freezing rate is increased to 1–10 °C/min. In this second freezing step, samples must be incubated in liquid nitrogen vapours (LNV) for 20 minutes. During this last stage, freezing devices containing sperm samples to be cryopreserved must be arranged so as to minimise temperature variations along the cryo-storage device. Finally, the sample is immersed directly in liquid nitrogen (LN) and is stored in LN tanks until its use.3,4
In slow freezing, freezing rates are controlled manually by the operator, so variations in the freezing rate are more likely to occur than in other freezing protocols (e.g., programmable freezing). Any variation in freezing rates is damaging but the cause of cryodamage can be different. When the freezing rate is above the optimal, there is greater mechanical injury because ice-crystal formation is promoted, whereas a below-optimal freezing rate promotes osmotic shock-induced damage.
Nevertheless, slow freezing is a cryopreservation protocol for human spermatozoa widely implemented in assisted reproduction.
Slow freezing allows the use of different cryostorage devices as sealed straws (volume capacity: 0.25–0.50 mL) or cryovials (volume capacity: 1.00–2.00 mL).5 Hence, it is a useful cryopreservation protocol to be used in the daily clinical routine as not only does it allow for the use of frozen samples, for example in a cryovial, for intrauterine insemination, but the cryostorage of straws for fertility preservation purposes can also be performed, meaning frozen samples from azoospermic or oncologic patients can be kept in multi-dose devices to be used in several further reproductive treatments.
Programmable Freezing
Programmable freezing aims to address one of the main weaknesses of slow freezing, providing a more strict control of freezing rates through the use of automated programmable LN freezers.6 Therefore, for programmable sperm freezing, sperm samples mixed with cryoprotectants are arranged on a platen before the freezing rate is selected. Samples are frozen first using a freezing rate of -1.5 °C/min, decreasing the temperature from 20 to -80 °C. Next, the freezing rate is increased to -6 °C/min. Once the temperature decrease is finished, samples will be submerged in LN (-196 °C).4
The main advantage of using programmable sperm freezers is the reproducibility and accurate control of the freezing rates. In contrast, its usefulness is limited to those circumstances when a high number of samples are required to be cryopreserved at the same time. Furthermore, in some circumstances programmable sperm freezing has been described as a less efficient process because of latent heat leading to delays in freezing rates, thus being detrimental for spermatozoa.5
Rapid Freezing
Rapid freezing, similarly to slow and programmable freezing, requires the addition of cryoprotectants. Rapid freezing is characterised by the direct contact of the mix of semen sample and cryoprotectants with LNV, consisting of a single freezing step. Firstly, semen samples are mixed with cryoprotectants. The homogenised mix is then incubated in LNV, causing a rapid decrease of temperature (-50 to -400 °C/min), prior to submerging the samples in LN tanks.4 The freezing rate during this protocol depends on two main factors: the period that the samples are incubated, and also the distance to LNV. This fact complicates control of the freezing rate. Therefore, the implementation of rapid freezing in andrology laboratories deserves a prior in-house study in which both previously mentioned variables are tested, so the proper time and distance to LNV can be established. One of the strengths of rapid freezing is the minimising of procedural time since only a single freezing step is needed, and it does not require high-technology devices.5
Dry Ice Freezing
Dry ice freezing is a cryopreservation protocol consisting of freezing the mixture of semen sample and cryoprotectants in dry ice pills. In order to do so, little drops of the combined mixture are placed in a dry ice block with sphere-like relief. In brief, once the semen sample is mixed volume to volume with cryoprotectants, little drops of the mixture are dispensed in the sphere-like wells of the dry ice block. Drops of the mixture are frozen within a minute, and finally all the drops of the same sample are placed in a tube and stored in LN (-196 °C).
Dry ice freezing is a rapid protocol that allows multi-dose storing in pills. Furthermore, thawing tests can be performed using a single drop to predict the quality of the thawed sample. Interestingly, this protocol does not require the control of freezing rates, since the freezing process is finished in 1 minute after the drops are placed in the sphere-like wells of dry ice. Therefore, dry ice freezing is a reproducible and easy-to-implement sperm cryopreservation protocol due to its cost-effectiveness.
The use of dry ice freezing for cryopreservation of human spermatozoa for assisted reproduction procedures was first described to freeze testicular human spermatozoa in 1996.7 Moreover, dry ice freezing has succeeded in cryopreserving human testicular spermatozoa from azoospermic patients who underwent gonadotoxic treatments.8 In addition, this cryopreservation technique is also used for sperm freezing in sperm donor programmes and for the sperm cryopreservation of infertile male patients.9-12
Non-Equilibrium Procedures: Vitrification of Human Spermatozoa
Sperm vitrification is characterised by the formation of glass-like solid structures preventing ice crystal formation. Sperm vitrification is a non-equilibrium cryopreservation procedure which achieves ultra-rapid freezing rates by means of submerging samples in LN (-196 °C), thus bypassing ice-crystal formation. The achievement of such ultra-rapid freezing rates requires a high concentration of cryoprotectants; hence, osmotic shock due to high levels of cryoprotectants is the main cause of cryopreservation-associated damage during sperm vitrification.
Prior to sperm vitrification, it is mandatory to perform a sperm preparation (or sperm selection) technique to remove seminal plasma, whilst during sperm freezing techniques preparation can be performed either before freezing or after thawing, as will be further discussed. Different sperm vitrification studies agree that seminal plasma is likely to present cell detritus, leukocytes, or even other sorts of micro-organism that would promote reactive oxygen species (ROS) production. Therefore, in order to prevent ROS-induced potential damage to sperm cells, it is recommended to perform a sperm preparation technique (swim-up or DGC) to remove the seminal plasma.
Traditionally, sperm vitrification is divided in two categories: aseptic and non-aseptic vitrification, depending on the direct contact of spermatozoa with LN.
Moreover, as a result of the increasing number of studies focussed on developing a cryoprotectant-free vitrification,13-16 vitrification protocols can also be sorted according to whether the use of cryoprotectant is required or not.
Vitrification of human spermatozoa is a fast protocol and easy to implement in andrology laboratories since it does not require complex equipment. However, it is not a technique widely implemented in human clinical practice due to its weaknesses. The high cytotoxic concentration of cryoprotectants and risk for cross-contamination as a result of direct contact with LN are minor disadvantages that could be improved by choosing aseptic cryoprotectant-free vitrification. Unfortunately, one of the major limiting factors for implementing sperm vitrification in the common clinical practice is the lack of acceptable reproductive outcomes when large volumes of samples are vitrified.
Regardless of all elegant and promising research reporting live births after the vitrification of human spermatozoa,15,17 it is not the preferred routine sperm cryopreservation protocol. Nevertheless, there is a growing tendency to implement and improve vitrification as a cryopreservation technique for testicular spermatozoa.18
Even though the main characteristics of cryopreservation techniques are important factors to be aware of, a major fact that should be considered is the sperm survival rate after thawing. Hence, assessment of sperm survival and sperm viability in thawed samples is crucial in choosing the cryopreservation technique to be used in clinical practice.
Table 2 summarises the vast majority of relevant comparative studies focussed on the assessment of sperm survival in thawed samples when different cryopreservation protocols are performed. As can be concluded from the results of these comparative studies, slow freezing offers the best outcomes, in terms of sperm quality after thawing, when compared with other freezing protocols.19-22 Regarding dry ice freezing, additional comparative studies are needed to compliment and elaborate on the evidence reported in Table 2.23
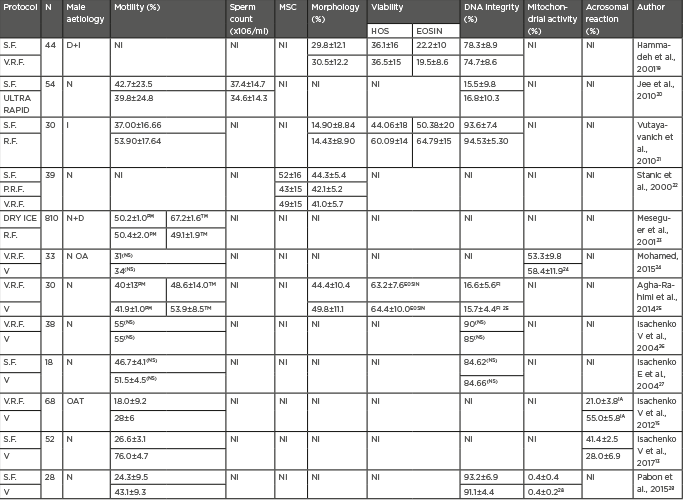
Table 2: Comparison of outcomes regarding sperm quality among different cryopreservation protocols.
D: sperm donors; EOSIN: Eosin-Nigrosin; FI: in reference to fragmentation index; HOS: hypoosmotic sperm swelling; I: Infertile patients; IA: in reference to intact acrosome (%); MSC: motile sperm concentration; N: normozoospermia; NI: not included; NS: no significance; OA: oligoasthenozoospermia; OAT: oligoasthenoteratozoospermia; PM: progressive motility; TM: total motility; S.F: slow freezing; R.F: rapid freezing; V: vitrification; V.R.F: liquid nitrogen vapor rapid freezing.
Regarding vitrification outcomes, it is still a debatable issue that vitrification offers better survival rates than freezing techniques. Studies have indicated no significant differences in sperm parameters between vitrified and frozen spermatozoa,24,25 although some authors suggest vitrified samples exhibit increased sperm motility after warming.15,26-29
Therefore, after considering cryopreservation outcomes, in combination with the technical characteristics of each cryopreservation technique, it can be concluded that the utility of vitrification as a sperm cryopreservation option to be used routinely in clinical practice is still lacking evidence, since robust differences between sperm freezing and vitrification, in terms of the sample quality after cryopreservation, cannot yet be established. In contrast, slow freezing offers good thawing outcomes, in terms of sperm quality, as has been reported in the comparative studies summarised in Table 2. Despite the lack of more comparative studies including dry ice freezing, the numerous experiences of Meseguer et al.8-11 in cryopreserving human sperm samples by dry ice freezing must not be dismissed given their previous outcomes in oncological patients and donors. Besides this, slow freezing and dry ice freezing have technical characteristics that facilitate their implementation as a routine sperm cryopreservation techniques for clinical purposes.
Optimisation of Human Sperm Freezing Protocols
The main reason sperm freezing protocols are preferable to vitrification in the daily clinical routine is not the achievement of acceptable survival rates, but the possibility to cryopreserve a higher number of spermatozoa, which is a major challenge facing clinicians today (Table 1B).20,30
It is agreed in the literature that a dramatic decrease in sperm count and motility is reported after thawing.31-33 Indeed, a decrease in motility as significant as 50% after thawing has been reported.34
Freezing After Sperm Preparation
Several studies agree to the idea of freezing prepared sperm samples, so sperm selection techniques are performed prior to cryopreservation with the aim to improve survival after thawing. Performing a sperm selection, or preparation, technique prior to sperm freezing implies both the removal of seminal plasma and the selection of a potential subpopulation of spermatozoa exhibiting better sperm parameters, such as motility.
Seminal plasma is the medium in which spermatozoa are contained in the ejaculate and is a mixture of different substances secreted by different organs, such as the testicles, epididymis, seminal vesicles, and Cowper glands. Decapacitating factors; antioxidant species; amino acids; lipids; proteins; uric acid; citric acid; bicarbonate; ions such as sodium, calcium, chloride, or magnesium; sugars such as glucose or fructose, and even metabolic derivates such as lactic and pyruvic acids are the main components of human seminal plasma.
Under physiological conditions, seminal plasma is removed from ejaculated spermatozoa during sperm transport in the female reproductive tract, specifically through the cervical mucus, uterus, and finally the fallopian tubes. This is to raise the capacitated status by means of undergoing several changes, such as the acrosomal reaction, so as to acquire the fertilising capability to penetrate the zona pellucida and to succeed in fertilising the oocyte.31
Swim-up and DGC are the two main in vitro sperm preparation techniques routinely used in clinical practice to select the best subpopulation of spermatozoa, and is performed prior to using a sperm sample for assisted reproduction purposes. Hence, prepared sperm samples are enriched either in morphologically normal spermatozoa or progressive motile spermatozoa when DGC or swim-up, respectively, are performed. The swim-up technique is one of the most commonly used sperm preparation techniques performed to select spermatozoa exhibiting better motility.33
Studies performing sperm preparation before freezing to remove seminal plasma are based on the premise that in the seminal plasma several compounds can be found, such as dead spermatozoa, debris, and leukocytes that induce ROS production, all of which can induce damage to sperm cells. Hence, these studies suggest that sperm damage is minimised when a subpopulation of spermatozoa, exhibiting the best sperm parameters, are selected and all potential damaging compounds are removed.31,33,35 Consequently, Esteves et al.31 and Petyim et al.33 suggested selecting for increased progressive motility (PM) rates by swim-up when sperm samples are prepared before the freezing step in normozoospermic patients. More interesting is the study carried out by Brugnon et al.,35 where another sperm preparation technique, DGC, is performed either before or after sperm freezing. One of the strengths of this work is that oligoasthenoteratozoospermic patients were included. Unfortunately, this study has some flaws in its design due to each study group including different patients, so aliquots from the same semen samples are not used in each cryopreservation protocol.
Freezing Before Sperm Preparation
In this protocol, sperm selection is performed after sperm freezing and leads to the selection of the best spermatozoa in the thawed sample, while cryoprotectant agents are also removed during sperm preparation in order to allow use in the following assisted reproduction technique. Several studies propose performing the sperm selection after freezing as a protocol that leads to an increase in the total amount of spermatozoa with good motility.33
Furthermore, evidence demonstrating the protective role of seminal plasma on sperm cells during cryopreservation is reported in the literature. As has been previously mentioned, seminal plasma is a physiological secretion of different compounds by several glands and organs of the male reproductive tract, thus constituting the ideal medium for sperm preservation prior to capacitation. In the seminal plasma different substances exist that act as protective factors against ROS release. Antioxidant enzymatic systems such as catalase, glutathione peroxidase, and superoxide dismutase, as well as other non-enzymatic compounds like ascorbic acid, E vitamin, carotenoids, and ubiquinones, are found in the seminal plasma. Moreover, Martiniz-Soto et al.36 suggest that poly-unsaturated fatty acids, present not only in the sperm cell plasma membrane but also in the seminal plasma, contribute to the antioxidant capacity of the seminal plasma, and also, particularly, to cryoresistance by means of increasing fluidity of the sample.36
Moreover, the physical adsorption of proteins present in the seminal plasma to the sperm cell surface helps to prevent temperature shock-induced damaged. These proteins are spatially arranged amongst the surface of sperm cell membrane, hence damage to sperm cell membrane during cryopreservation is minimised.37 Similarly, in other studies it is suggested that heparin binding proteins also have a protective function against temperature shock, preventing lipid peroxidation which could contribute to ROS production.38
In this sense, Donnelly et al.32 support the strategy of freezing before sperm preparation, since selecting spermatozoa after thawing leads to better PM rates. The research carried out by this group goes further by demonstrating that the addition of seminal plasma substantially increases motility rates in samples prepared previously to sperm freezing.32 Donnelly et al.32 did not assess other types of motility, such as total motility (TM), and also sperm count, even though a computer-assisted sperm analyser was used.
A more recent study also agrees with the findings observed by Donnelly et al.,32 as they succeeded in demonstrating that the total amount of viable spermatozoa is maximised when sperm preparation is performed after freezing,39 since not only PM and TM, but also total motile sperm count (TMSC) are improved. TMSC combines TM with sperm concentration, so it is an estimator of the number of available motile spermatozoa, regardless of the type of motility they exhibit. This fact is especially relevant when sample availability is limited. Thus, considering male patients with impaired sperm parameters singularly, TMSC lowers to reach critical values when sperm selection is performed prior to cryopreservation.39 As a foregone conclusion to what has been commented above, sperm freezing before selection must be particularly recommended to sub-fertile male patients, since it is the protocol that ensures a higher number of motile spermatozoa. In addition, recent evidence highlights TMSC as an accurate estimator of sperm viability, being a faster and simpler method that avoids staining and incubation steps associated with classical sperm viability tests such as eosin staining or the hypo-osmotic swelling test.40
Table 3 summarises the most relevant comparative studies regarding sperm freezing before or after sperm preparation.
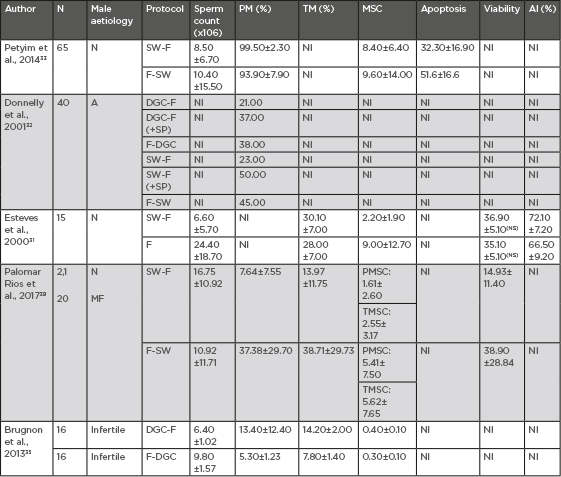
Table 3: Sperm freezing before or after sperm preparation.
A: asthenozoospermia AI: acrosomal integrity; DGC: density gradient centrifugation; F: freezing; MF: male factor subfertility; MSC: motile sperm count; N: normozoospermia; NI: not included; NS: no significance (p>0.005); PM: progressive motility; SP: seminal plasma; SW: swim-up preparation technique; TM: total motility; TMSC: total motile sperm count.
As can be concluded from the results in Table 3, sperm selection performed either by swim-up or DGC prior to sperm cryopreservation seems to offer better motility rates in the thawed samples.
However, sperm freezing prior to sperm preparation reports better outcomes, in terms of motile sperm count. Hence, sperm preparation after sperm freezing yields higher numbers of motile spermatozoa. When TMSC is calculated from the data derived from the studies carried out by Petyim33 and Esteves,31 it is found to be higher when sperm preparation is performed after sperm freezing, although they suggested an improvement in sperm motility when freezing previously prepared sperm samples. Therefore, it could be concluded that sperm preparation after sperm freezing is the protocol that leads to higher values of TMSC.
Clinical Outcomes After Using Fresh or Cryopreserved Human Spermatozoa for Assisted Reproduction Technologies
The main objective of ART is to achieve a healthy baby. It is therefore important to consider the techniques used for cryopreservation and the clinical results available when aiming to achieve optimum outcomes from ART, especially when compared to fresh samples.
Table 4 includes clinical outcomes, in terms of fertilisation, pregnancy, and delivery rates, of different comparative studies in which both fresh and cryopreserved spermatozoa were used.41-58
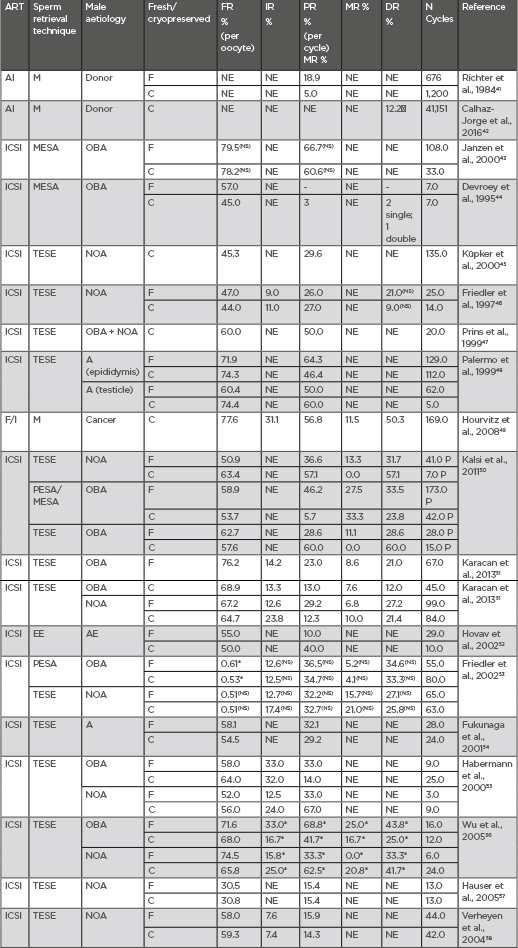
Table 4: Clinical outcomes after the use of frozen semen.
*statistically significant (p<0.005). : Higher than AI with own not cryopreserved semen; A: azoospermia; AE: anejaculation; AI: artificial insemination; ART: assisted reproduction technique; C: cryopreserved; CUM: cumulative clinical pregnancy rate; DR: delivery rate; EE: electroejaculation; F: fresh; F/I: IVF/ICSI; FR: fertilization rate; ICSI: intracytoplasmic sperm injection; IR: implantation rate; M: masturbation; MESA: microsurgical epididymal sperm aspiration; MR: miscarriage rate; NI: not included; NOA: non-obstructive azoospermia; NS: not significant (p>0.005); OBA: obstructive azoospermia; PESA: percutaneous epididymal sperm aspiration; PR: clinical pregnancy rate; TESE: testicular sperm extraction.
As it can be deduced from the outcomes in Table 4, the use of either fresh or cryopreserved semen has no significant effect on clinical outcomes. Indeed, the use of cryopreserved semen samples in azoospermic patients undergoing surgical sperm retrievals has advantages since it avoids repeated surgical intervention, for instance through testicular, microsurgical epididymal, or percutaneous epididymal sperm aspiration. Furthermore, it is also reported that acceptable clinical results are achieved in male fertility preservation programmes when cryopreserved sperm samples from oncologic patients are used.49
Of note, differences cannot be established regarding the further development of offspring when fresh or cryopreserved semen samples are used. Hence, the use of cryopreserved semen samples for assisted reproduction purposes is a safe practice which neither increases the malformation rate nor worsens further physical development when compared with the use of fresh semen.59








