Abstract
Transferring embryos that are most likely to successfully implant and develop is important in optimising the efficiency of assisted conception. Slow-freezing of spare embryos has a high attrition rate; thus, actively selecting a viable embryo for a fresh transfer can theoretically result in a superior cumulative live birth rate compared to a conventional assessment of morphology. However, with vitrification and its much lower attrition rate, active selection may not deliver an improved cumulative live birth rate, as more viable embryos may be excluded due to the limitations of the technique than are lost due to warming attrition. For some women, the principal benefits of active selection techniques are likely to be associated with a reduction in the number of miscarriages and a reduced time to achieve a successful pregnancy or start another stimulated cycle. Active selection procedures need to be safe, accurate, and effective, without jeopardising the chance of a live birth. The analysis presented in this paper shows that, from the perspective of a self-funding woman, adding a costly active selection option is entering into a lottery for a better result that is most likely to offer no advantage and even the possibility of an inferior outcome for some. Gauging willingness-to-pay to avoid miscarriage and to reduce treatment time is likely to be complex, and depends on who is making the decision and how they are counselled. Evaluating cost-effectiveness, for which the unit of health is one live birth, is unlikely to be helpful in supporting a case for public funding or private insurance for a better selection technique. The author of this paper explores the theoretical potential of active embryo selection to optimise a full cycle of assisted conception, with particular reference to single embryo transfer.
INTRODUCTION
Effective embryo selection and successful cryopreservation of spare embryos are important to optimise the efficiency of a stimulated cycle of assisted conception.1 The cryopreserved embryo survival rate has improved with the use of the newer vitrification technique over slow-freezing,2,3 and the process now has clinical outcomes comparable to fresh transfer for ovulatory women.4 Successful selection of viable embryos offers the potential to achieve a healthy singleton live birth event following the fewest possible number of transfer procedures and to reduce the risk of miscarriage. It is now possible to transfer embryos one at a time without jeopardising the chance of pregnancy and avoiding clinical complications associated with multiple gestations.1,3,5
Abnormal embryo morphology is often associated with genetic abnormalities, and culturing embryos for as long as possible allows many unsuitable embryos to arrest naturally.6 Active selection of surviving embryos may involve morphological, developmental, and genetic criteria that could have some value in differentiating viable and non-viable embryos prior to transfer.7,8 Advances in technology offer increasingly more effective and objective assessment of embryo viability. Hot topics include time-lapse systems for embryo imaging9,10 and preimplantation genetic testing for chromosome aneuploidy (PGT-A) using array comparative genomic hybridisation or next-generation sequencing;11,12 however, what constitutes appropriate validation and implementation into routine practice is the subject of much debate,13-15 and implementation and uptake of these technologies vary worldwide.
The aim of this article is not to evaluate the numerous technologies and approaches to assessing embryo viability at different development stages, but to explore the theoretical potential of active embryo selection to optimise a full cycle of assisted conception, with particular reference to single embryo transfer.
EFFECTIVENESS OF ACTIVE EMBRYO SELECTION IS SENSITIVE TO PREDICTIVE VALUE AND CRYOPRESERVATION EFFICIENCY
Using a previously published model,16 Figure 1 shows the hypothetical effect of cryopreserved embryo survival on the cumulative live birth rate (CLBR) for women with two blastocysts suitable for transfer compared to a more effective selection method (test) with conventional morphological assessment. From the test perspective, the negative predictive value (NPV) is the proportion of normal (negative) test results that correctly predict a live birth, and the positive predictive value (PPV) is the proportion of abnormal (positive) test results that correctly predict no live birth. It is postulated that embryo selection is more effective than morphological assessment alone when using PGT-A for every chromosome with a genetic microarray (Φ: NPV 41.4%, PPV 96.0%),16 and would be even more effective with a better test (Φ‘: NPV 62.5%, PPV 97.2%).16 The incidence of viable embryos is estimated to be 25.4%, and the clinical pregnancy miscarriage rate to be 8.5% without genetic testing and 5.1% following genetic testing. Putative non-viable embryos with an aneuploid test result are excluded from a fresh or subsequent warmed transfer.
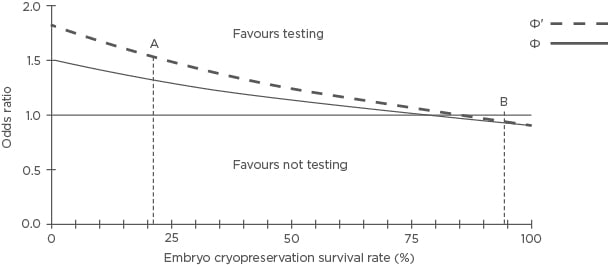
Figure 1: The hypothetical effectiveness of active selection versus a conventional embryo morphology assessment and cryopreservation survival on the cumulative live birth rate for women with two embryos suitable for transfer or testing.
Blastocyst survival rates: 21.4% (A) and 94.5% (B).
Φ: NPV 41.4%, PPV 96.0%; Φ’: NPV 62.5%, PPV 97.2%; NPV: negative predictive value; PPV: positive predictive value.
Adapted with permission from Scriven.16
Using intact blastocyst survival rates of 21.4% (A) and 94.5% (B) for slow-freezing and vitrification, respectively,17 and compared to a standard morphological embryo assessment, per 100,000 women it is estimated that:
- PGT-A Φ with slow-freezing: 6,040 (35,529 versus 29,489) more women could have a live birth, with 828 (1,903 versus 2,731) fewer clinical miscarriages and 30,042 (85,916 versus 115,958) fewer single embryo transfer procedures.
- PGT-A Φ‘ with slow-freezing: 9,654 (39,143 versus 29,489) more women could have a live birth, with 634 (2,097 versus 2,731) fewer clinical miscarriages and 53,329 (62,629 versus 115,958) fewer transfers.
- PGT-A Φ with vitrification: 1,997 (41,355 versus 43,352) fewer women could have a live birth, with 1,798 (2,216 versus 4,014) fewer clinical miscarriages and 70,463 (100,005 versus 170,468) fewer embryo transfer procedures.
- PGT-A Φ‘ with vitrification: 1,744 (41,608 versus 43,352) fewer women could have a live birth, with 1,785 (2,229 versus 4,014) fewer miscarriages and 103,895 (66,573 versus 170,468) fewer transfers.
A sufficiently accurate test increases the likelihood of selecting a viable embryo for a fresh transfer, avoiding the relatively high attrition associated with slow-freezing, resulting in a superior CLBR compared to conventional assessment. However, a test that is substantially better at selecting viable embryos for transfer can be inferior for live birth when combined with vitrification because relatively more viable embryos are excluded due to incorrect, abnormal (false-positive), or inconclusive results than are lost due to warming attrition. A warming survival rate of 94.5% may be considered modest; however, Figure 1 shows that higher rates have a negligible effect on the odds ratio (OR).
Since the unit of health is one live birth, adding an active selection test, which results in fewer live births than using conventional morphological assessment, cannot be cost-effective.16 However, there are likely to be fewer clinical miscarriages because most aneuploid embryos will be excluded from transfer, and fewer transfers are likely to reduce the time required to complete a full cycle for some women; gauging the willingness-to-pay is likely to be complex and depends on who is making the decision.
INSIGHTS FROM A HYPOTHETICAL CLINICAL TRIAL
In the UK, the current National Institute for Health and Care Excellence (NICE) guidelines covering diagnosing and treating fertility problems in the UK recommend a single embryo transfer (fresh or cryopreserved) in the first full in vitro fertilisation (IVF) cycle for women aged <37 years, and more if there are ≥1 top- quality embryos for women aged 37–39 years.18 In theory, although not always in practice, state funding is available for IVF, excluding the addition of PGT-A.
Figure 2 and Table 1 show the hypothetical outcomes from a virtual trial19 conducted to provide insight into the cost-effectiveness of incorporating PGT-A into a first treatment attempt for every woman <40 years old (median age: 33 years; range: 22–39 years) to achieve a first live birth delivery. Fresh and vitrified-warmed embryos (if available) were transferred one at a time in a first complete full cycle (no dropout), comparing selecting out embryos (exclusion from transfer of putative aneuploid embryo) and ranking-only (transferring putative euploid embryos first and no exclusion) with conventional morphological assessment without additional testing. It is assumed that the vitrified-warmed embryo survival rate is 94%, and the NPV and PPV of the genetic test are 40.4% and 95.2%, respectively, with an incidence of 29.4% viable transferable embryos. With only conventional assessment, the live birth rate per transfer was 28.0% and the clinical pregnancy miscarriage rate was 10.0%; it took up to 43 months to complete a full cycle with up to 10 transfer procedures. Maternal age is an important independent predictor of live birth and in the study a younger woman (<38 years old) had a 2.6-times (p<0.0001) higher chance of a live birth than an older woman (38–39 years old). Costs are based on UK pound sterling 2017 prices:19 IVF cycle (£3,300), intracytoplasmic sperm injection (£900), stimulation drugs (£900), embryo cryopreservation and storage (£800), warmed embryo transfer (£1,400), drugs (£150), and PGT-A (£2,950). The study was powered to detect a 40% reduction (3.6% versus 6.0%) in the cumulative clinical miscarriage rate (CCMR) (single-sided, 80% power, alpha 5%). An ideal scenario is also considered where only a viable embryo (if available) is transferred first with no risk of miscarriage, which has the optimum CLBR of 65.2%.
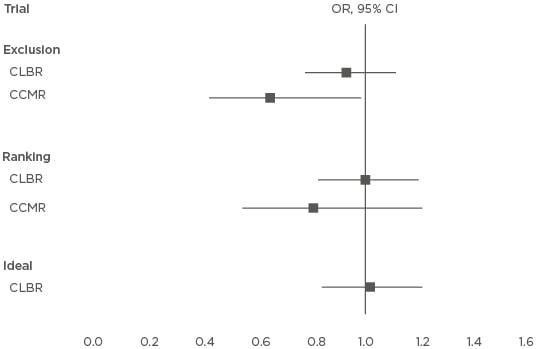
Figure 2: Results from a virtual trial and the hypothetical effect of incorporating preimplantation genetic testing for chromosome aneuploidy into a first treatment attempt for every woman <40 years old and the active selection on the intention-to-treat cumulative live birth and clinical miscarriage rates when transferring single embryos at a time.
CLBR: OR >1 favours testing; CCMR: OR <1 favours testing.
CI: confidence interval; CLBR: cumulative live birth rate; CCMR: cumulative clinical miscarriage rate; OR: odds ratio.
Adapted with permission from Scriven.19
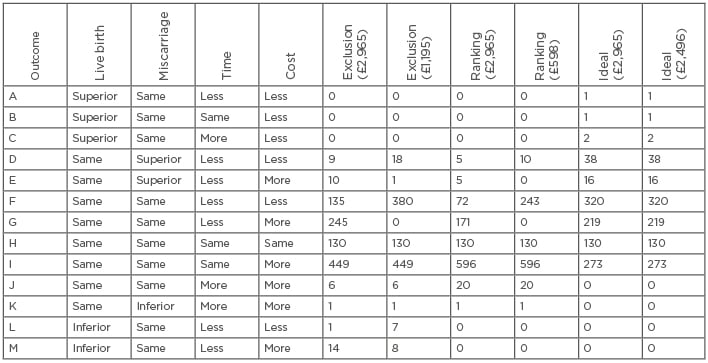
Table 1: Hypothetical outcome permutations (A-I) for 1,000 women from a virtual trial illustrating the cost-effectiveness of incorporating preimplantation genetic testing for chromosome aneuploidy into first treatment attempts for every women <40 years old to achieve a first live birth.
Adapted with permission from Scriven.19
Considering only the first transfer attempt odds, a woman had a 1.699-times (39.8% versus 28.0%; p<0.0001) and 1.614-times (38.6% versus 28.0%; p<0.0001) higher chance of a live birth using exclusion and ranking-only, respectively, indicating that PGT-A is more effective than conventional morphological assessment to select viable embryos. Women who achieved a clinical pregnancy had a 0.728-times (7.5% versus 10.0%; p=0.2643) and 0.726-times (7.4% versus 10.0%; p=0.2598) higher chance of clinical miscarriage with exclusion and ranking- only, respectively. Including cryopreserved embryos, the CLBR was similar with PGT-A (exclusion: 63.3%, ranking: 64.8%) and without genetic testing (64.8%); however, exclusion was more effective to avoid clinical miscarriage (CCMR: 3.7% versus 5.6%; OR: 0.648; p=0.0451) (Figure 2).
Data from the published virtual trial19 are summarised in Table 1. The number of women with different permutations (A–I) of outcome are presented, comparing PGT-A to a conventional morphological assessment, including measures for their superiority for a live birth and or clinical miscarriage, and time and cost. Two costs for genetic testing were used for each transfer strategy: the median 2017 price for PGT-A (£2,965) and the PGT-A cost required to make genetic testing no more expensive overall, including all 1,000 women intending to start treatment (£1,195 for exclusion and £598 for ranking-only).
For exclusion (£2,965 for genetic testing), 15 (1.5%) fewer women achieved a live birth due to rejection of viable embryos with a false- positive result (L and M). Nineteen (1.9%) women avoided clinical miscarriage (D and E), of whom 9 (0.9%) completed their cycle more quickly with reduced expense (D). Testing increased the time and expense for 7 (0.7%) women (J and K) due to the incorrect exclusion of viable embryos. A total of 130 (13.0%) women, had no embryos suitable for transfer or testing (H), which includes 15 cycles abandoned before oocyte retrieval. For 694 (69.4%) women, the cycle outcome was the same for live birth and clinical miscarriage but with greater expense (G and I); however, the cycle time was reduced for 245 women (G). For 135 (13.5%) women, the outcome for live birth and clinical miscarriage was the same, but in a shorter time period (median reduction: 6 months, range: 6–15 months) and less expensive (median reduction: £135, range: £135–£5,585) (F). Reducing the PGT-A cost to £1,195 marginally increased the number of women for whom testing was superior for miscarriage with less time and reduced expense from 9 (0.9%) to 18 (1.8%) (D). However, the number of women for whom the live birth and miscarriage outcome was the same but with reduced time (median reduction: 3 months, range: 3–15 months) and cost (median reduction: £355, range: £355–£7,355) was substantially increased from 135 (13.5%) to 380 (38.0%) (F).
Ranking-only was not inferior for live birth (L and M) but avoided fewer clinical miscarriages than exclusion (D and E) and reduced the time and expense for fewer women (D and F). The number of women for whom the cycle cost was more expensive with genetic testing with the same outcome was increased from 449 (44.9%) with exclusion to 596 (59.6%) with ranking only (I).
The ideal test produced a superior live birth outcome for four (0.4%) women due to a fresh transfer avoiding cryopreservation attrition (A, B, and C); two of whom took more time due to a full-term gestation period. An optimal 54 (5.4%) women avoided a clinical miscarriage (D and E), of whom 38 (3.8%) were associated with less time and expense; however, assuming no overall difference in the total cost (£2,496 cost for genetic testing), 273 (27.3%) women had the same outcome with greater expense (I).
From the perspective of the self-funding individual, adding PGT-A is likely to be a costly lottery. Neither exclusion nor ranking-only of embryos should be expected to be superior for live birth, and the former strategy is likely to disadvantage some women. A very small minority of women are likely to benefit by avoiding a clinical miscarriage and, while a larger minority are likely to benefit by reducing the time to complete their cycle, most women are likely to pay more for the same outcome. However, some may have reduced costs associated with travel and accommodation if fewer visits to the assisted conception unit are required (not included in the analysis).
If it were possible to reduce the cost of a more effective active selection method, such that the overall total cost was the same as a conventional assessment, then a more persuasive case for public funding or private insurance might be made. Reducing the CLBR by excluding viable embryos incorrectly and transferring embryos with an abnormal (although possibly incorrect) result using ranking-only is likely to have some bearing on the willingness-to-pay. From the perspective of society, testing may also afford direct and indirect cost savings associated with managing fewer miscarriage and prenatal diagnosis procedures, and the upbringing of offspring with congenital disability (not included in the analysis). Potential savings associated with multiple pregnancy, preterm, and neonatal complications are unlikely to be significant since only one embryo is transferred at a time.16
CLINICAL TRIALS WITH CUMULATIVE OUTCOMES
Clinical prospective intention-to-treat embryo selection studies, including fresh and cryopreserved embryos, are likely to be costly, take years to complete, and have a risk of obsolescence. The European Society of Human Reproduction and Embryology (ESHRE) study into the evaluation of oocyte euploidy by microarray analysis (ESTEEM)20,21 is a multicentre, randomised, double-blind trial with an intention-to-treat analysis of PGT-A with array comparative genomic hybridisation on polar bodies including women aged 36–41 years. The trial started in February 2012 and was completed in September 2017, but the final report has not yet been published. Initial results were presented at the ESHRE Annual Meeting in July 201722 and, following the randomising of 396 women, the study showed that the likelihood of a live birth within 1 year was not increased (41 versus 42 women with at least one delivery), but was achieved with fewer transfers using selection (178 versus 270).23 The proof-of-principle study20 that preceded the trial concluded that the ploidy of the zygote can be predicted with acceptable accuracy. Based on the pilot study, with 67.7% prevalence for aneuploidy the PPV (the proportion of abnormal test results that are aneuploid) and the NPV (the proportion of normal test results that are euploid) were estimated to be 94% (likely to be between 89% and 97%) and ~100% (likely to be better than 93%), respectively,24 with a substantial proportion (>10%) of normal zygotes likely to be excluded incorrectly.
Randomised controlled selection trials that attempt to estimate the CLBR are few. A recently published study,25 which includes older women aged 38–42 years and excludes poor prognosis patients, reported a significantly higher live birth rate in the tested group following the first transfer: 52.9% (36 of 68) versus 24.2% (23 of 95) (OR: 3.522 [1.804–6.873]; p=0.0002), which is indicative of effective active selection of viable embryos. However, when adding live births from cryopreserved embryo transfers during the 6 months following the study recruitment period, the cumulative delivery rate in the tested group is similar: 37.0% (37 of 100) versus 33.3% (35 of 105) (OR: 1.175 [0.662–2.085]; p=0.5285). It is not clear how many women who had not achieved a live birth during the study period still had cryopreserved embryos available. Fewer transfers were required to achieve a live birth and the time to pregnancy was reduced. It also seemed likely that around one in five women may avoid a miscarriage, although this may be an optimistic estimate.19
CONCLUSION
The advent of vitrification for embryo cryopreservation has changed the landscape of assisted reproduction. The principal benefits of active selection techniques are likely to be associated with a reduction in the number of miscarriages, and a reduced time to achieve a successful pregnancy or start another stimulated cycle. Active selection procedures need to be safe, accurate, and effective for these outcomes without jeopardising the chance of a live birth. Evaluating cost-effectiveness, where the unit of health is one live birth, is unlikely to be helpful in supporting a case for public funding or private insurance for a better active selection technique. Gauging willingness-to-pay to avoid miscarriage and reduce treatment time is likely to be complex and to depend on who is making the decision and how they are counselled. From the perspective of the self-funding individual, adding a costly active selection option is entering into a lottery for a better result, which may offer no advantage for most women and the potential for a worse outcome for some.








