The current gold standard of embryo evaluation is based on morphology and morphokinetics, however there have been many efforts to design non-invasive tests to assess embryo viability,1 including imaging of embryo metabolism by fluorescence lifetime imaging microscopy and high-resolution nuclear magnetic resonance for metabolic biomarker detection. The authors have previously demonstrated that Matrix Assisted Laser Desorption Ionisation Time of Flight (MALDI-ToF) mass spectrometry offers an immediate, sensitive, and straightforward analysis of spent embryo culture fluid to identify proteins present.2 This approach can now be used to confirm pregnancy outcome retrospectively and give a confirmation of embryo viability within minutes.3 In this study, the authors asked if a non-invasive mass spectrometry-based tool could be used to identify embryos that result in ongoing pregnancy.
A retrospective cohort study was carried out which included 1,190 spent media samples from embryo cultures collected from a single in vitro fertilisation clinic in the USA. The samples were collected between March 2014 and March 2018. The outcomes were intrauterine pregnancy, no implantation, biochemical pregnancy, and spontaneous abortion. Furthermore, outcomes from genetic testing for aneuploidy resulting in euploid and aneuploid results were also found. Only spectra from fresh, single transfer embryos with clearly positive (n=57) or negative (n=38) outcome were used in the development of the tool.
Upon receiving frozen embryos, the embryo culture media was thawed, diluted, and analysed using MALDI-ToF mass spectrometry in a laboratory in the UK. Analysis was performed using CHCA matrix, with spectra obtained in a mass range of 200–2,000 m/z. Data files were subject to semi-quantitative computational analysis using Python programming language. Raw data was pre-processed using a fully automated and fast computational approach previously developed for urine and further adapted to culture media data to render comparable analysis.4
The Blastocyst and Embryo Screening and Selection Tool (BESST) was developed following the analysis of embryo culture media from a single, fresh transfer embryo culture media. The previously developed automated computational workflow was applied to generate a reference spectral pattern of ideal embryo profile with chosen samples.4 Criteria for sample selection included highest quality scores as determined by Blastocyst Quality Score; a numerical blastocyst-morphology grading system; ongoing pregnancy outcome; and preimplantation genetic testing for aneuploidies.
Similarities to the reference pattern were computed, based on peak positions and intensity values, assigning a score for each embryo. The resulting complex, non-linear scores were subject to cluster analysis and subsequently mapped into five distinct classes 0–5 with continuous numerical values, which can be interpreted linearly. With this method, the authors were able to identify 76.9% of ongoing pregnancy cases for embryos that score >4, while a score of <1.5 predicts that the embryo has a 35.7% chance of ongoing pregnancy (Figure 1).
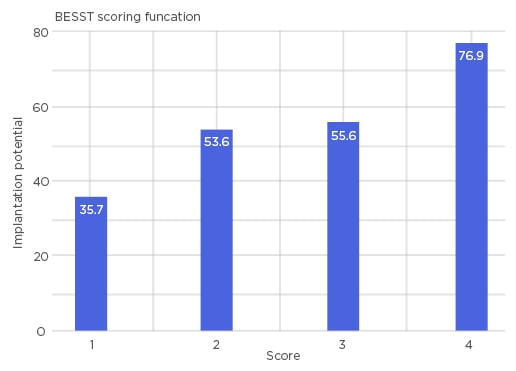
Figure 1: Bar chart representing four distinct scores of BESST.
A higher score indicates a higher chance of successful pregnancy (76.9%) and a lower score indicates a lower chance of successful pregnancy (35.7%). BESST: blastocyst and embryo screening and selection tool.
It is important to note that results of or close to 100% cannot be achieved due to an underlying confounding factor in which there are cases of unreceptive endometrium, regardless of the quality of the embryo. In light of this consideration, the implantation potential of 76.9% shown here is a favourable result.
When applied prospectively, immediately prior to embryo transfer, the BESST test could enable the objective, consistent, and non-invasive analysis of the likelihood of achieving ongoing pregnancy and, compared to morphology alone, could be optimised to see improvement in pregnancy rates for any clinic using the tool.