Abstract
Aim: The estimation of the ‘dry weight’ in a patient on haemodialysis with end-stage renal disease is an important clinical challenge to date. Physical examination has its limitations in the precise assessment of volume status. The monitoring of blood volume, natriuretic peptides, and bioimpedance spectroscopy are explored as a guide for the ultrafiltration process during haemodialysis (HD) therapy. Unfortunately, none of these methods has shown promising results when used in isolation and has serious limitations. The point-of-care lung ultrasonography has emerged recently as an adjunct to physical examination as a non-invasive, radiation-free technique to estimate extravascular lung water. In this study, the authors aimed to compare the volume status assessment in end-stage renal disease patients on HD using conventional clinical methods, bio-electrical impedance, and chest ultrasound (US).
Materials and Methods: A prospective cohort study was conducted on 34 patients undergoing regular HD in the Department of Nephrology dialysis centre at the University College of Medical Sciences Guru Teg Bahadur Hospital, Delhi, India, a multi-speciality tertiary care centre. Parameters included to assess the dry weight of patients were bio-impedance spectroscopy and chest US, measured in two phases: 30 minutes before and 10–60 minutes following the HD session.
Results: A total of 100 assessments were done on 34 patients over 6 months. The mean pre-HD extracellular water was 17.52±2.69 L and post-HD was 16.38±2.46 L, showing a significant reduction (<0.001). The bioimpedance analysis showed that 44% of the volume status assessments had fluid overload (≥1.1 L), even when the patients were considered to be in a state of clinical euvolemia, while 79% of the assessments had a Comet Score of ≥3 suggesting a fluid overload state. Most assessments showed a significant reduction in the number of B-lines (i.e., 62% [Comet Score of between 0–2]). The mean post-HD Comet Score was 1.73±1.36 (37%).
Conclusion: Chest US to assess Comet Score is highly correlated with the clinical signs and symptoms. Lung Comet Scores can also be highly correlated with ultrafiltration volume, and thus can be used as a good marker for achieving dry weight in dialysis patients.
Key Points
1. Over- and underestimation of dry weight for a prolonged time has a significant impact on morbidity and mortality in patients on haemodialysis (HD).
2. Chest ultrasound is the most recent method in clinically detecting extravascular lung water and estimating dry weight in patients with end-stage renal disease on HD.
3. The present study added weight to the role of chest ultrasound in lung water measurement in patients on HD, and compared it with body fluid volume status.
INTRODUCTION
Estimation of reliable and reproducible dry weight in patients with end-stage renal disease on haemodialysis (HD) is a clinical challenge to date. ‘Dry weight’ is the targeted post-HD weight where the patient is as close as possible to their normal hydration state, without having any symptoms indicating over- or under-hydration at or after HD treatment. Clinical estimation of the dry weight in patients is done by a trial-and-error method, correlating it with a history of conditions resulting in extracellular volume overload (increased sodium intake) or depletion (excessive sodium losses in conditions like persistent diarrhoea) and their related symptoms like dyspnoea, postural dizziness, cramps, and headache. Clinical signs such as blood pressure (BP) with postural changes, weight with its interdialytic changes, neck veins congestion, and oedema provide an evaluation of fluid status.1 However, evaluating patients clinically alone is not accurate enough. This degree of imprecision is reflected in the intradialytic symptoms of chronic volume overload, which results in poor control of BP.2 Hence, other methods, including biochemical markers (natriuretic peptides such as atrial natriuretic peptide and B-type natriuretic peptide), bioimpedance analysis (BIA), inferior vena cava diameter and its collapsibility, have been developed over time for the better assessment of the accurate fluid status; however, these methods have several shortcomings, such as poor specificity for natriuretic peptides, poor correlation with extravascular lung volume as compared to total body water (BIA and inferior vena cava diameter and collapsibility). Hence, no method is considered a gold standard, and a combination of two or more methods should be used for a more accurate assessment of fluid status.1
Overestimation of dry weight for a prolonged period, without any preventive measure, may result in hypertension, left ventricular hypertrophy, and heart failure (HF). On the other hand, underestimation of dry weight is responsible for chronic dehydration, leading to the development of hypotension.3 Both these complications significantly impact morbidity and mortality in patients on HD. Therefore, it is obvious that using better assessment methods, which are easy to conduct and cost-effective, for determining volume changes during HD is needed.
A lung ultrasonography (USG) is the most recent method being utilised for the detection of extravascular lung water. The US beam is reflected by sub-pleural thickened interlobular septa (a low impedance structure surrounded by air with a high acoustic mismatch) in presence of excessive lung water. This US reflection generates hyperechoic reverberation artefacts between the thickened septa and the overlying pleura known as ‘lung comets’, or B-lines.4 Lung comets are defined as vertical artefacts that arise from the pleural line and then extend to the edge of the screen, which moves synchronously with respiration.
The dynamics of B-lines were first evaluated by Noble et al.5 in patients undergoing HD. They reported a real-time decrease in these artefacts when the volume was removed and suggested the use of this technique in the assessment of euvolemia.5 Mallamaci et al.6 further investigated this relationship between lung US findings, status of body fluids volume, and echocardiographic parameters in patients on HD. They found a strong association between the lung US and altered left ventricular functions, but no association with hydration status was assessed by bioimpedance.6 Trezzi et al.7 reported a significant correlation between fluid overload before and after HD, and weight loss with the number of B-lines7.
In this study, the authors aimed to evaluate the feasibility of lung water measurement by chest US in patients on HD and compare the lung water and body fluid volume status, as assessed by a clinical assessment, chest US, and bioimpedance spectroscopy (BIS). The secondary objective of their study was to test the regression of lung comets (B-lines) according to fluid removal in patients on HD.
MATERIAL AND METHODS
This cross-sectional study was conducted at a dialysis centre in the Department of Nephrology at the University College of Medical Sciences Guru Teg Bahadur Hospital, Delhi, India, a multi-speciality tertiary care centre. The sample size was computed as n=34 using the calculation for the proportion of the hospital-based population, with a confidence interval of 95%; an expected prevalence of 40%; a power of 80%; an error of 5%; acceptable absolute and relative precision of 6%; and p=0.05. Written informed consent was taken. All patients received a conventional intermittent 4-hour dialysis, using low permeability polysulfone membranes with a standard bicarbonate dialysate. Prescribed weight was taken from the patient logbook and estimated by the attending nephrologist based on clinical criteria such as weight, BP, and presence of oedema or vascular congestion.
Inclusion and Exclusion Criteria
Inclusion criteria for cases were that the patients were on bi-weekly maintenance HD, were of either gender, and over the age of 18. Patients on once- or thrice-weekly HD; had lung diseases such as pulmonary fibrosis; Stage III and IV of dyspnoea according to the New York Heart Association Functional Classification (NYHA) showing HF, which could affect the results of lung US regardless of the hydration; pacemakers or implantable electronic device; or major amputations of extremities were excluded. A conventional intermittent 4-hour dialysis was given to all patients, using a low permeability polysulfone membrane with a standard bicarbonate dialysate. Data collection was done on a pre-designed proforma. The rate of ultrafiltration was prescribed clinically, based on the interdialytic weight gain in comparison to the targeted weight by the treating nephrologist. Assessment of the weight was done by the attending nephrologist based on clinical criteria like weight, BP, oedema, or vascular congestion, and compared with the prescribed weight taken from the patient logbook. The dry weight assessment parameters, including BIS and chest US were measured twice: 30 minutes before and 10–60 minutes after the HD session.
A gap of at least 1 month was kept in case more than one assessment is done for a single patient. Informed written consent was taken from all the enrolled participants.
DATA COLLECTION
Data collection was done per the pre-designed proforma blinded to the ultrafiltration rate, as prescribed clinically according to the interdialytic weight gain in comparison with the target weight by the treating nephrologist. This included demographic parameters of the patient; measurements of BP in the supine position after a 10-minute rest using an aneroid sphygmomanometer with documentation of an episode of intradialytic hypotension (systolic BP: <90 mmHg; diastolic BP (DBP): <60mmHg); weight being assessed with an electronic scale, height being assessed with conventional measures, and BMI being calculated using the Quetelet Index (weight [kg]/height [m2]).
Bioimpedance Analysis
QuadScan 4000 Touch (Bodystat, Douglas, Isle of Man) was used to measure calf BIA. BIS was performed 30 minutes before HD and 10–60 minutes after the HD session by making the patient lie down in bed. The wrist of the contralateral arms of the arteriovenous fistula and the homolateral ankle was used for the placement of electrodes. Fluid overload was defined as the difference between extracellular water (ECW) as measured by BIA pre- and post-dialysis (ECW BIA pre-dialysis-ECW BIA post-dialysis). Requirements before performing BIA included fasting for 4–5 hours the patient was to refrain from any physical exercise a minimum of 12 hours, caffeine (i.e., tea, coffee, and energy drinks), and consumption of alcohol for 24 hours.
Chest Ultrasound
Chest US was performed by the same radiologist, who has over 20 years of experience in chest US. The MicroMaxx® Ultrasound System (Sonosite, Bothell, Washington, USA) with a 6 MHz vascular probe was used for the imaging to look for an alveolar-interstitial syndrome characterised by the presence of specific artefacts called B-lines or ‘comet tails’. The presence of comet tails was proof of ling congestion caused by fluid overload in patients on HD.8 US was performed in the supine position. A longitudinal scan in the left hemithorax (second to the fourth intercostal space) and right hemithorax (second to the fifth intercostal space) at the parasternal, midclavicular, anterior axillary, and midaxillary lines of each side in a total of 28 sectors was performed. Hyperechogenic linear artefacts that were emerging from the pleural line, up from the bottom of the screen, and coherent with respiratory movements were defined as the B-lines. The Comet Score was determined by the total sum of the B-lines found in each examined site, which reflected the extent of extravascular accumulation of the fluid in the lung.
Study Variables
Definitions
Dry weight was defined as the weight estimated by the attending nephrologist and based on clinical criteria such as weight, BP, the presence of oedema, or vascular congestion. The accumulated weight was defined as the subjective dry weight gain, while weight loss was defined as the difference between the pre-and post- dialysis weight changes. Residual weight was defined as the difference between obtained weight after dialysis and the subjective dry weight.
Impedance results
The reference values calculated using impedance for fluid overload to define euvolemia in the normal population were within the range of -1.1 –1.1 L.9 Patients were considered dehydrated if the fluid volume was less than the reference value (-1.1 L) and euvolemic if the volume of fluid was within the reference range (±1.1 L); they were considered to be in in overload if the fluid volume was greater than the reference value (1.1 L).
Results of lung ultrasound
Pulmonary congestion by fluid overload in patients was defined with the following characteristics:10 multiple artefacts per scan (minimum three artefacts) and bilateral positivity.
Clinical Outcomes
The average ultrafiltration rate was calculated as follows
Ultrafiltration rate (mL/h/kg)=(pre-HD weight–post-HD weight [kg])×1,000 (mL/kg)
Session duration (h)×post-HD weight (kg)
Statistical Analysis
Data analysis was done using SPSS Statistics version 21.0 (IBM, Armonk, New York, USA). Categorical variables were presented as numbers and percentages, while continuous variables were presented as mean±standard deviation and median. Quantitative variables were presented using the Student’s t-test/Mann–Whitney U test and the qualitative variables were correlated using the χ² test. The Pearson correlation coefficient was used to correlate quantitative variables with each other. A p value of <0.05 was considered statistically significant.
RESULTS
This cross-sectional study was conducted over 6 months to determine the efficacy of chest US in the assessment of body fluid volume status in patients with chronic kidney disease (CKD) on dialysis. A total of 100 assessments were done on 34 patients over the 6 months. There were 8 (23%) females and 26 (76%) males for whom chest US and BIS were performed pre- and post-dialysis (Table 1). Most patients (12 [61.7%]) were aged 51–60 years. The mean age of the study population was 54.8 years. The oldest patient was 80 years old. The mean height of the patients was 1.66 ±0.07 m and BMI 22.66 ±3.66 kg/m2. BMI was calculated from the pre-dialysis weight using the Quetelet Index (weight [kg]/height [m2]). Most of the patients, (24 [70.5%]) had normal body weight (BMI: 18.5–24.9 kg/m2) and five (14.7%) were underweight (BMI: <18.5 kg/m2).
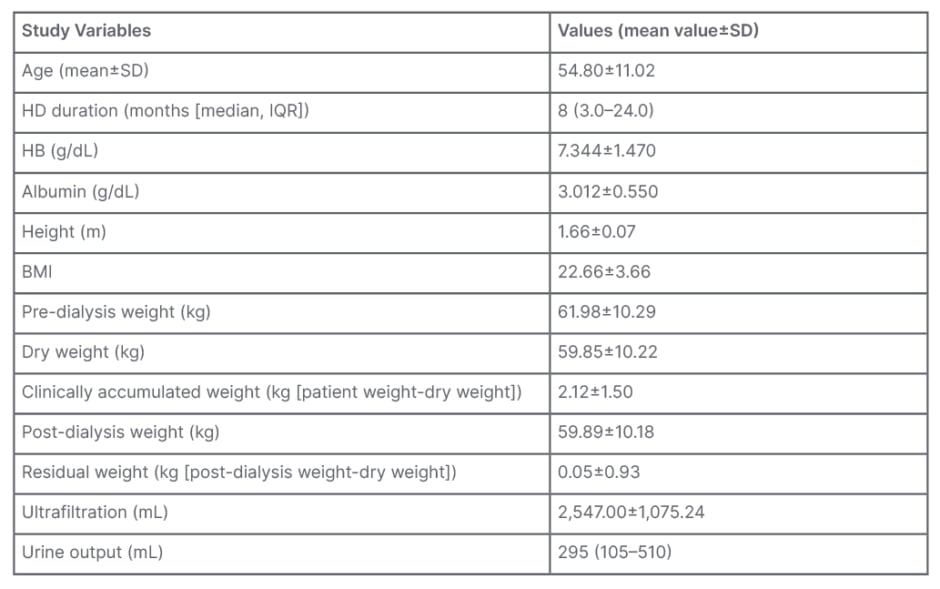
Table 1: Demographics and clinical profile of the studied population.
HD: haemodialysis; IQR: interquartile range; SD: standard deviation.
Of the authors’ patients, 97% had hypertension and 62% had BP readings above 140/90 mmHg. Intradialytic hypotension (BP: <90/60) was found in 3% of the assessments. Diabetes was found in 18 (52%) of the patients, while five (15%) had coronary artery disease and three (9%) had chronic obstructive pulmonary disease. A total of 85% of patients showed no limitation of ordinary physical activity (NYHA Stage I) while 15% showed restriction of ordinary physical activity (NYHA Stage II).
Table 1 depicts the mean values of the patients’ pre-dialysis weight, dry weight, and clinically accumulated weight were 61.98±10.29 kg, 59.85±10.22 kg, and 2.12±1.5 kg, respectively. Post-dialysis weight and residual weight were 59.89±10.18 kg and 0.05±0.93 kg, respectively. Pre-dialysis weight was between 50-70 kg in 76% of the assessments. Dry weight was assessed by trial-and-error methods using various clinical parameters. Figure 1A depicts that the dry weight was between 50–70 kg in 73% of assessments. Clinically accumulated weight was between 1–3 kg in 47% of assessments and 8% of the patient’s assessments showed that there was no weight gain seen during the inter-dialysis period. A total of 72% of the assessments had a post-dialysis weight between 50–70 kg. Approximately 53% of assessments showed significant residual weight, while 47% of assessments showed no residual weight.
Figure 1B depicts that the mean pre-HD ECW was 17.52±2.69 L and post-HD was 16.38±2.46 L, showing a significant reduction (p<0.001). A total of 44% of the volume status assessments, as per BIA, showed fluid overload (≥1.1 L) even when the patients were considered to be in a state of clinical euvolemia. A total of 79% of the assessments had a Comet Score of ≥3, suggesting a fluid overload state. The mean pre-HD Comet Score was 4.54±2.53. A significant reduction in the number of B-lines was seen with most of the assessments (i.e., 62% had a Comet Score of between 0–2). Figure 1C depicts the mean post-HD Comet Score, which was 1.73±1.36. Of the 100 assessments, 37% had significant B-lines (≥3 per scan) and were considered to be in fluid overload, even after achieving a state of clinical euvolemia.
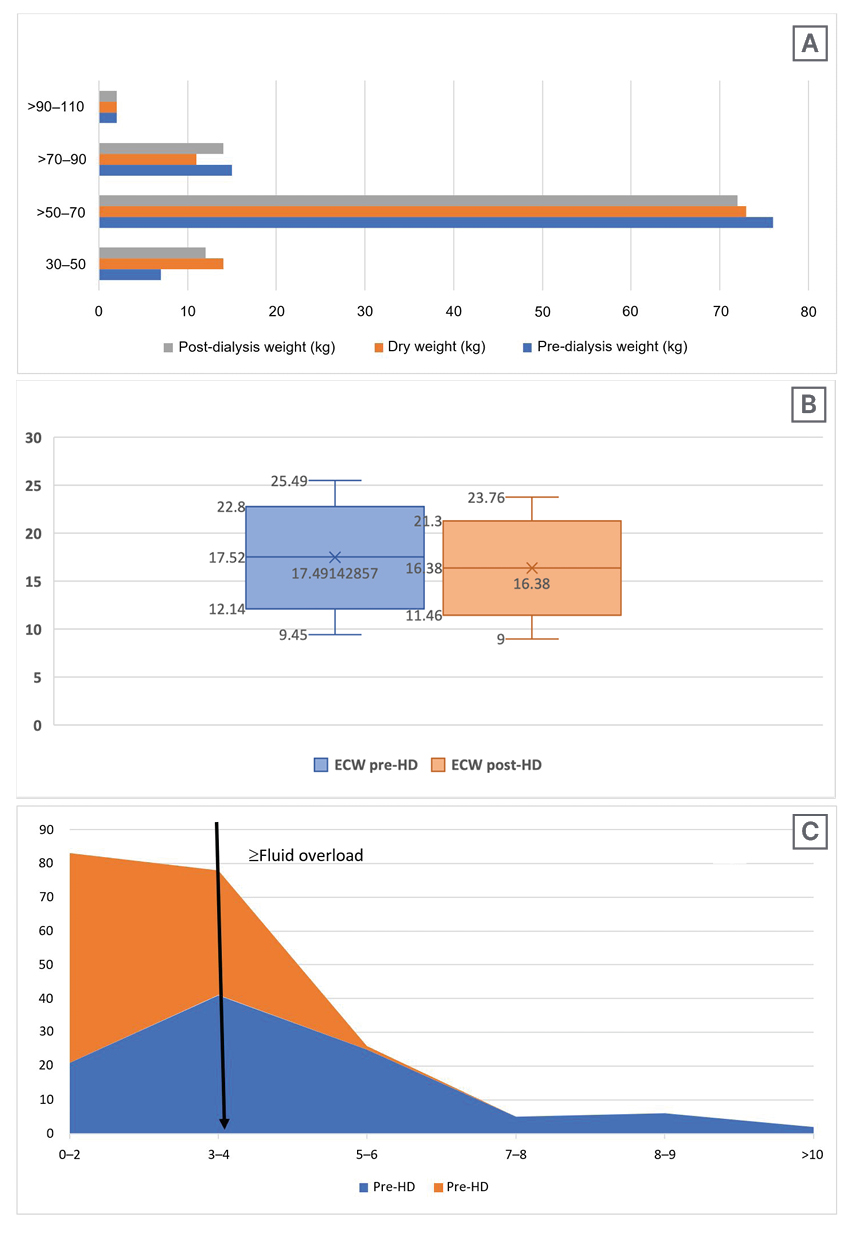
Figure 1: Analyses of patients pre- and post-haemodialysis.
A) Pre- and post-HD and dry weight recordings of patients with CKD. B) Mean ECW measurement on BIA pre- and post-HD. C) Pre- and post-HD Comet Score on chest US.
BIA: bioelectrical impedance analysis; CKD: chronic kidney disease; ECW: extracellular water; HD: haemodialysis; US: ultrasound.
Figure 2 depicts the correlation between residual weight by clinical methods, fluid overload by BIA, and ultrasonography (USG) chest Comet Score post-dialysis. There was a significant correlation between fluid overload as per BIA and USG chest Comet Score post-HD (p<0.001; r2=0.75), while no significant correlation could be seen between residual weight, as assessed by clinical methods and fluid overload as per BIA or USG chest Comet Score post-HD (p=0.2763 and 0.1972, respectively; r2= 0.0056 and 0.0101, respectively).
Figure 2: Post-haemodialysis Comet Scores in correlation to clinical residual weight and fluid overload, as well as clinical residual weight and fluid overload.
A) Correlation between post-HD Comet Score (chest USG) and clinical residual weight in kg (post-HD weight-dry weight). B) Correlations between post-HD Comet Score (chest USG) and fluid overload (ECW BIA pre-HD-ECW BIA post-HD). C) Correlation between clinical residual weight in kg (post-HD weight¬-dry weight) and fluid overload in L (ECW BIA pre-HD-ECW BIA post-HD).
BIA: bioelectrical impedance analysis; ECW: extracellular water; HD: haemodialysis; USG: ultrasonography.
DISCUSSION
Assessment of reliable and reproducible dry weight in patients on HD is a challenging clinical task. The authors’ study aimed to compare the volume status assessment in patients with CKD on HD using conventional clinical methods, bioelectrical impedance, and chest US. A total of 100 assessments were done on 34 patients with CKD and biweekly maintenance HD.
In the authors’ study, there was a male predominance (76%). Similarly, studies by Bardai et al.,3 Torino et al. (65.0%),11 Vitturi et al. (63.3%),12 and Allinovi et al.13 (60.8%) on bedside lung US for volume status assessment in patients on dialysis had a male predominance, with a male:female ratio of 1.1:1.0. Torino et al.11 in their LUST study found that 65% of the patients were males.
In the authors’ study, hypertension was the most common comorbidity seen among patients with CKD (97%), followed by diabetes (52%), coronary artery disease (CAD [15%]), and chronic obstructive pulmonary disease (9%). Similarly, studies by Kuzuhara et al.14 and Youssef et al.15 showed that hypertension was the commonest comorbidity seen among patients with CKD, accounting for 41 (83.7%) and 30 (75.0%) cases, respectively. Other studies by Fraser et al.16 on comorbidity burden in patients with CKD also reported predominance of hypertension (1,528 [87.8%]), followed by diabetes (294 [16.9%]), CAD (398 [22.9%]), and respiratory disease (181 [10.4%]).
In this study, high BP (≥140/90 mmHg) was found in most patients during the pre-dialysis period (i.e., 62% of the assessments). Similarly, a study by Rahman et al.17 reported high BP in 87.7% of the patients. In another study by Yang et al.18 on the paediatric population, hypertension was observed in 11 (47.8%) of patients. Recommendations from the Kidney Disease Outcomes Quality Initiative (KDOQI) suggest that decreasing fluid overload in the interdialytic period can help in better control of BP in patients on dialysis.19
In the authors’ study, intradialytic hypotension (BP: ≤90/60 mmHg) was found in three patients. Out of the three patients, two had diabetes and hypertension, while one had underlying CAD. One patient also had a low pre-dialysis BP recording (<90 mmHg). In another study by Sands et al.20 intradialytic hypotension was found in 17.8% of treatments, with a higher prevalence in patients with diabetes and lower pre-dialysis systolic BP.
In this study, most of the assessments show a pre-dialysis weight between 50–70 kg (76% of patients). The mean pre-dialysis weight was 61.98±10.29 kg, while studies by Raimann et al.21 and Passauer et al.22 reported a mean pre-dialysis weight of between 75.70±16.20 kg and 75.00±18.00 kg, respectively. Dry weight in most of the assessments was between 50–70 kg (73%). The mean dry weight was 59.85±10.22 kg. Similarly, Alexiadis et al.23 found the mean dry weight of patients to be 73.28±16.50 kg in their study; however, in a study on fluid overload in patients on HD, Antlanger et al.24 found a mean dry weight of between 75.09±19.20 kg.
In the author’s study, 47% out of 100 assessments had a clinically accumulated weight of between 1–3 kg (pre-dialysis weight-dry weight). On the other hand, 8% showed no weight gain. The mean clinical accumulated weight was 2.12±1.50 kg. Similarly, Vitturi et al.12 reported a clinically accumulated weight of between 2.60±1.50 kg.
Patients with greater interdialytic weight gain had higher DBP readings in the authors’ study. Similarly, Ipema et al.25 reported a higher DBP with a higher interdialytic weight gain, which is an independent risk factor for cardiovascular mortality.
In this study, most of the assessments had post-dialysis weights between 50–70 kg (72%). The mean post-dialysis weight was 59.89±10.18 kg. However, the mean post-dialysis weight in a study by Basso et al.26 and by Maduell et al.27 was significantly higher (71.40±13.60 kg and 69.50±14.80 kg, respectively).
In the authors’ study, the mean residual weight was 0.05±0.93 kg. Similarly, Bardai et al.3 reported the mean residual weight to be 0.56±1.18 kg in their study. The mean pre-dialysis ECW on BIA in the authors’ study was 17.52±2.69 L and 16.38±2.46 L post-HD (p<0.001). On the other hand, Papakrivopoulou et al.28 and Yang et al.18 reported a mean ECW of between 14.04±0.23 and 7.80±3.40 L pre-dialysis, respectively, and a mean ECW of between 13.21 ±0.21 and 7.20 ±3.30 L, respectively. There is a positive correlation between weight loss and percentage reduction in ECW. Patients with higher serum urea, albumin, creatinine, potassium, and normalised protein nitrogen appearance had greater ECW fall on univariate analysis. In context, with other reports of patients with some residual renal function and on diuretics showed smaller changes in ECW.29
In the authors’ study, 56% of the assessments showed patients to be euvolemic as per BIA, while 44% were hypervolemic (≥1.1 L). Significant fluid overload (≥1.1 L) was associated with higher BP recordings in 32 out of 62 assessments.
Similarly, Bardai et al.3 found that 38% of the patients were in a fluid overload state as per BIA, post-dialysis. Wizemann et al.,30 in their study on risks of overhydration in patients with CKD, found that an overhydrated state is an independent predictor of hypertension and, therefore, mortality on long-term dialysis is second only to diabetes.
The mean pre-HD Comet Score in the authors’ study was 4.54±2.53, while the post-HD Comet Score was 1.73±1.36 (p<0.0001). Similar findings were reported by Vitturi et al.,12 with a mean pre- and post-HD Comet Score of between 3.13±3.40 and 1.41±2.47, respectively.
In this study, 23% of assessments had significant lung congestion in the post-dialysis period, even when patients were considered to have clinical euvolemia. Similar results were seen in a study by Bardai et al.,3 where 20% of the patients had significant lung congestion post-dialysis. On the contrary Basso et al.,26 found that 15 (65%) of the patients had significant pulmonary congestion after dialysis. The exclusion of patients having severe dyspnoea (NYHA Stages III–IV) was the cause of the lower Comet Scores in the authors’ study, while relatively higher Comet Scores in other studies was secondary to patients having decompensated HF. Depending on the pathologic conditions, there will be differences in the density and distribution of BIA. Thickened subpleural interlobular septa are represented by the dispersed septal syndrome. Alveolar flooding, also known as an interstitial-alveolar syndrome and white lung, is a more severe variant of interstitial lung disease.31 Gas exchange is compromised, and the dyspnoea gets worse in the later stages. According to the NYHA’s functional classification, the relationship between the Comet Score and the degree of dyspnoea makes logical sense.
The authors’ study was cross-sectional, and the sample size is not large enough to give results that may be representative of the general population. Device validity was not confirmed using the tracer dilution technique.
CONCLUSION
Chest US performed at the bedside is now emerging as a reliable, easy-to-apply, and safe method for measuring lung water and intravascular overload and their reduction after dialysis, even in asymptomatic patients. Comet Score, assessed on chest USG, is highly correlated with the clinical signs and symptoms; it even precedes the development of symptoms in patients on HD. Moreover, lung Comet Score is also highly correlated with ultrafiltration volume and thus, can be used as a good marker for achieving dry weight in patients on dialysis. These observations strongly support the use of lung US in estimating volume overload and monitoring the response to therapy in patients on HD. Furthermore, the authors’ findings also conclude that the lung US Comet Score is superior to clinical methods in assessing the volume status in patients on HD and, hence, the target dry weight for these patients.







