INTRODUCTION
Assessment of the ovarian morphology is one of the most commonly performed ultrasound examinations. Polycystic ovarian syndrome (PCOS) is a multifactorial, multifaceted, polygenic disorder with varying phenotypes. It defines a labyrinthine symptomatology including menstrual cycle irregularities, hormonal imbalance, and metabolic disturbance. Historically, this syndrome has been diagnosed clinically with supportive lab parameters. However, the role of ultrasound has mutated from identifying, to mis-defining and finally to re-classifying PCOS.1-4 At present it seems that the ultrasound identification of the ‘string of pearls’ has cemented this disease with a misleading name. A supposed increase in the detection of polycystic ovarian morphology on ultrasound has been accredited to advances in technology allowing better visualisation of the ovaries/stroma/follicles by higher frequency probes with the possibility of endovaginal imaging. Nevertheless, there is a disparity in what the ultrasound shows, how the clinician interprets the report, and what the patient understands about her diagnosis. Identification of the multifollicular ovary is still quite frequently ascribed to PCOS, while ovarian ultrasound remains ambiguous to the different phenotype of PCOS. Whether morphological disparities represent a normal variation in ovarian anatomy or true precursors of PCOS remains debatable. The absence of definition of a ‘normal’ ovary with respect to volume and follicular number, makes the diagnosis of PCOS more challenging.5,6
Over time, ovarian volume remains the most reliable, reproducible and sensitive method for identification of PCOS (figure 1). However, it has a lower diagnostic accuracy due to considerable overlap with normal women. Confusion prevails in the setting of pelvic infection, hormonal treatment, and ethnic variability. In the setting of poor image resolution, whether due to use of lower frequency probes or patient habitus, volume remains the best usable criterion.3,6 While endorsing the Rotterdam criteria, the recent 2018 International Evidence-Based Guidelines also acknowledged the fact that ultrasound criteria are evolving, and new thresholds need to be established. This development is accredited to both accelerated development in technology, as well as increased availability of ultrasound in widespread populations. However, it should be mentioned that technical skill varies widely, and as such it’s important to realise that it is not only the development of ‘defined criteria’, but also distribution of skill and expertise among practitioners, which will determine the diagnosis of PCOS at a community level.4
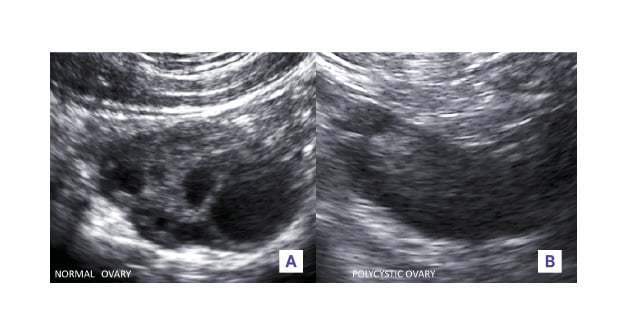
Figure 1: A) Normal versus B) polycystic ovaries (B mode, transvaginal).
The normal ovary is smaller in size, has few randomly distributed follicles of varying sizes interspersed within the stroma. In contrast in a typical polycystic ovary, both the length and width are increased as well as the ovarian area. The follicle number, with a diameter mainly between 2 and 5 mm, is more than 12. The distribution within the ovaries is mainly peripheral. The increased and hyperechoic stroma occupies the centre of the ovaries.
Assessment of the number of follicles has been upheld to be one of the specific features of PCOS. The concept is to sweep through the entire ovarian volume and count the number of follicles in each ovary in totality, keeping in mind not to measure sonolucencies <2 mm, as they do not represent actual follicles (figure 2). Grid systems, tagging, and marking have been used in post-processing to accurately measure the follicular number per ovary (FNPO) in order to improve reliability and reproducibility. However, these methods are time-consuming and not widespread. Though there have been documented ethnic variations, generally PCOS patients are seen to have a higher number of follicles per ovary. The FNPO has been found to be the best describing feature in cases of unilateral PCOS. The distribution of follicles has also been proposed to help identify ‘classic PCOS’, though its accuracy remains in doubt. Disordered follicular growth and recruitment has been identified using ultrasound as well. Transvaginal ultrasound allows a superior assessment of follicles than transabdominal ultrasound, and should be utilised whenever possible. However, in circumstances involving cultural or personal barriers, transabdominal remains the only modality widely accepted.3,4,6-11
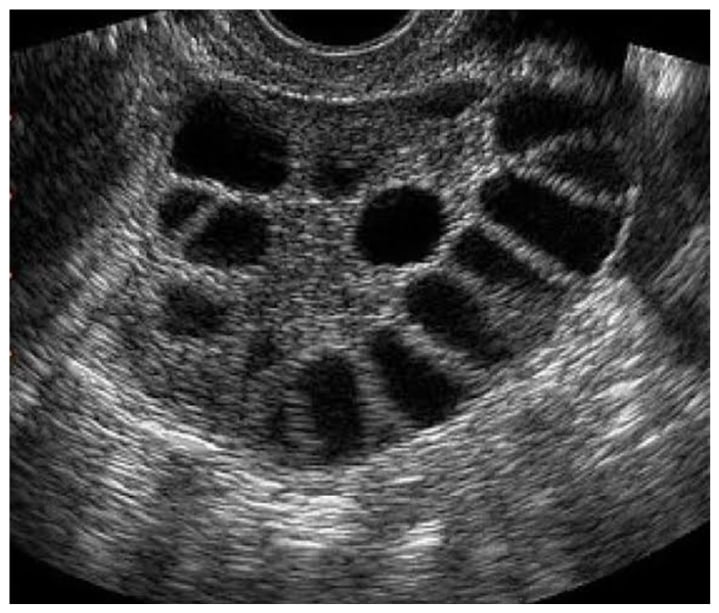
Figure 2: A multifollicular ovary (B mode, transvaginal).
It shows multiple antral follicles predominantly 4-10 mm in size, more so in a random distribution without predominance of stroma.
Bright, echogenic stroma has been subjectively accredited to PCOS. There have been many efforts to correlate qualitative indexes of stromal echogenicity with PCOS; however, it has been found that the intrinsic echogenicity of the ovarian stroma is no different in PCOS than in the normal ovary. ‘Feature analysis’ objectively measures the brightness, or echogenicity, of the ovarian stroma. This is done by measuring the intensity level of the ultrasound pixels within the stroma displayed on an ultrasonic image. The mean echogenicity of a given area can then be calculated. In a study by Buckett et al.,12 it was found that even though the stromal index was significantly elevated in polycystic ovaries, the mean stromal echogenicity was not different. The subjective ‘bright’ stromal echotexture in polycystic ovaries is attributed to a synergistic effect of increase in ovarian stromal volume, and hence a relative lower mean echogenicity of the entire ovary.6,12 The stromal/total are ratio has also been found to have high sensitivity and specificity, albeit with poor reproducibility(figure 3). With the improvement in ultrasound software, the brightness or echogenicity of the ovarian stroma can be determined much more objectively, and therefore, the quantification of ovarian stroma by computerised reading of ultrasound images has revealed that stromal hypertrophy is a frequent and specific feature in ovarian androgenic dysfunction, with some studies demonstrating that increased stromal volume correlates positively with serum androgen level.6,15 However, no standardised method exists for determining stromal volume. Because overall ovarian volume correlates well with stromal volume in polycystic ovaries, and is more easily measured in clinical practice, the determination of overall ovarian volume is a reliable surrogate for ovarian stromal assessment.6,13,14
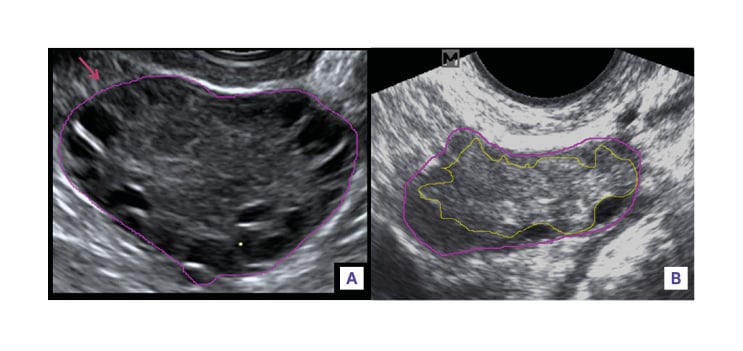
Figure 3: Measurement of ovarian/stroma ratio (B mode, transvaginal).
A) Ovarian area was measured by outlining the external limits of the ovary with an electronic caliper tool (pink). B) Stromal area was measured by outlining the peripheral profile of the stroma, avoiding antral follicles represented by anechoic structures in the ovary (yellow). The outline was extended to the periphery of the ovary when no follicles were present around that peripheral portion of the ovary.
Elevation of impedance indices of the uterine arteries has been described in patients with PCOS, though it seems a multitude of factors contribute to this finding, including co-existence of obesity. A higher pulsatility Index and systolic/diastolic ratio has also been described. Since most of these studies were performed on patients primarily concerned with infertility, it is not known where exactly these findings fit into the pathophysiology of PCOS.1,16-19
Three-dimensional ultrasonography (3D USG) allows accurate measurement of the stromal volume, follicular number, and ovarian volume. Accuracy is comparable to 2-dimensional USG, with ample agreement of the Rotterdam criteria. Although promising, 3D USG is relatively expensive, and not widely available.20,21
The imaging of ovaries on magnetic resonance is a new and exciting frontier. Ovarian volume on MRI has been shown to be quite sensitive for diagnosis, with high reproducibility. Peripheral follicular distribution and FNPO>28 is supportive, but not as reproducible. Though the advantages of MRI in obese patients with poor quality scans is obvious, MRI cannot be extrapolated to the entire ‘PCOS eligible’ population due to sheer number and cost.6,22 Artificial intelligence and convolutional neural networks form another exciting area which, however, may not translate to clinical practice soon or enough.23
What?
A specific protocol, as laid down by the 2018 International Guidelines, is quintessential to the proper evaluation of PCOS. This protocol should not be limited to this syndrome, but extended to all gynaecological ultrasound evaluations.4
When?
The ultrasound should be preferred to perform the scan on Day 2-7 of the menstrual cycle. This prevents any growing follicle from hiding smaller ones or modifying ovarian volume. In case of oligo or amenorrhoeic women, scanning may be performed at random, or 2-5 days after progesterone-induced bleeding.4
How?
Scanning should be done with an ‘optimally’ filled bladder, avoiding extremes in transabdominal sonography (TAS), and empty bladder in transvaginal sonography. Identify the ovaries in relation to iliac vessels. Entire ovary should be scanned in two orthogonal planes. Measurement of ovarian volume (length x width x thickness) should be done precisely, ensuring adequate visualisation of the ovarian contour. If possible, a follicular count should be obtained with careful meticulous sweeping of both ovaries individually. This count may not help in the diagnosis of a particular patient, but will help long-term to allow us to redefine criteria. If the setting allows, estimation of stromal area should be done offline. Additionally, it should be ensured to assess the liver and pancreatic fat grade, and have a look on the adrenal areas.4
Why?
One of the guidelines that stood out for the author, but is rather ignored in daily practice, is the ‘non-inclusion of ultrasound in diagnosis of PCOS in adolescents with gynaecological age of less than 8 years’. So why give ultrasound for this age group? It is not just PCOS that can cause pathology in this population. We also have to think beyond PCOS. The role of ultrasound varies with the patient age and primary concern, from dermatological troubles to fertility treatment, and needs to be tailored accordingly.4
Who?
A common core protocol should be followed by every person performing the ultrasound. Reporting needs to be standardised and uniform. Establishment of ethnic thresholds should be considered. It should be understood that the criteria for TAS and transvaginal sonography are not the same. The prioritisation of the volume criteria in TAS and low resolution/difficult scans is also to be acknowledged.4
The sheer number of patients requiring an ultrasound for evaluation of ovaries implores establishment of reliable, easy to follow, reproducible, and accurate ultrasound protocol. Radiologists as well as sonographers should undergo sensitisation so as to make the reporting of ovarian ultrasound uniform. Perhaps a large-scale normal ovarian morphology nomogram preparation is the need of the hour, with emphasis on differences in ethnic origin. Finally, it should be remembered that ultrasound is only a brushstroke in the masterpiece that is PCOS.