Abstract
Aims: Ultrasound (US) guided percutaneous splenic biopsies are uncommon and are ordinarily done in the context of an indeterminate splenic lesion with a probable underlying malignancy, and where there is no other safe accessible site to obtain tissue, or where the diagnosis of a splenic metastasis would impact management. The authors aimed to assess the diagnostic accuracy and complication rate of US-guided percutaneous biopsy of the spleen.
Materials and methods: The authors assembled a list of 43 biopsies between 2005–2023 at St. James’s University Hospital in Leeds, UK.
Results: Twenty-two biopsies (51%) were performed to assess progression in the context of known or previous malignancy, 22 were performed to assess for malignancy in patients with no prior malignant history, and the remaining six (14%) biopsies were conducted to assess for infection. Three patients had a prior splenic biopsy, and 30 experienced one needle pass. Thirty-seven of the 43 samples (86%) proved to be diagnostic. Positive samples included both benign and malignant abnormal splenic tissue. Malignant diagnoses included diffuse large B cell lymphoma, Hodgkin’s lymphoma, leukaemia, and metastases. Benign conditions included non-specific reactive changes, infection, sarcoidosis, and haemangioma. The authors established there were 30 true positives and zero false positives. There were five true negative biopsies and one false negative. The overall diagnostic yield and accuracy are 86% and 84%, respectively. There were no major complications and two minor complications; two patients developed perisplenic haematomas, which were conservatively managed.
Conclusion: The authors concluded that US-guided percutaneous splenic biopsy is a safe procedure with a high diagnostic yield and relatively high accuracy.
Level of evidence: Level 4, retrospective cohort.
Key Points
1. Ultrasound (US)-guided percutaneous splenic biopsy is not typically a first-line investigation for indeterminate splenic lesions due to complications such as haemorrhage.
2. A retrospective study conducted at the authors’ tertiary centre looked at every US-guided percutaneous splenic biopsy performed, including the indication of the biopsy, histopathology results, and complications.
3. US-guided percutaneous splenic biopsy and histopathological examination remain the best diagnostic options in certain cases. This study demonstrated it is a safe procedure with a high diagnostic yield and relatively high accuracy.
INTRODUCTION
Splenic abnormalities usually present as a focal lesion(s) or diffuse splenomegaly outside the context of trauma. Most splenic lesions are incidental and benign, such as a simple cyst or infarct, and therefore do not require further investigation. However, some may display malignant characteristics or present in the context of a known malignancy posing diagnostic uncertainty. Ultrasound (US)-guided percutaneous splenic biopsies are uncommon and are ordinarily done in the context of an indeterminate splenic lesion with a probable underlying malignancy (i.e., lymphoma), and where there is no other safe accessible site to obtain tissue, or where the diagnosis of a splenic metastasis would impact on management.1 Splenic biopsies are typically not a first-line investigation because of the perceived risk of haemorrhage as well as other complications such as pneumothorax, bowel perforation, and renal injury.1 Table 1 is a summary of indications and contraindications of US-guided percutaneous splenic biopsy. The laboratory parameters in Table 1 are utilised from the authors’ hospital’s guidelines for nonvascular interventional procedures.
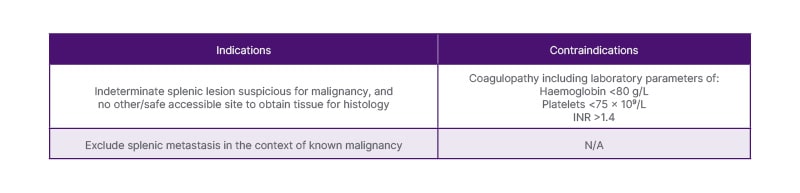
Table 1: Indications and contraindications of ultrasound-guided percutaneous splenic biopsy.
INR: international normalised ratio; N/A: not applicable.
AIMS
The authors aimed to assess the diagnostic accuracy and complication rate of US-guided percutaneous needle biopsy of the spleen.
OBJECTIVES
After reviewing the relevant literature, the authors identified the following parameters to measure: indication of biopsy, number of previous biopsies, number of needle passes, results of the biopsy, and complications. The results of the biopsy included whether the sample was diagnostic (i.e., of sufficient yield to allow analysis) and the histology. Abnormal splenic tissue was considered a positive biopsy, including benign conditions or reactive changes. Normal splenic tissue was labelled as a negative biopsy. False positives and negatives were described in cases where results were contradicted by biopsy of an organ other than the spleen and/or follow-up imaging. Complications were classified into major and minor using the guidelines produced by Omary et al.2 Major complications included haemorrhage requiring intervention (such as transfusion or embolisation), pneumothorax requiring chest drain, other significant intra-abdominal injury such as bowel perforation, and death.2 Minor complications included conservative haemorrhage management including overnight admission for observation.2
METHOD
The authors liaised with the Radiology Information Team at St James’s University Hospital, a tertiary centre in Leeds, UK, to provide them with a list of patients with every US request and report including ‘spleen’ and ‘splenic’. This data span from 2005–2023 and produced over 200,000 patients. They looked through the reports in retrospect via radiology imaging software and assembled a list of 43 US-guided splenic biopsies that were performed on 40 patients. Electronic medical records were reviewed for histology results, post-biopsy complications, and follow-up. Paediatric patients, percutaneous aspirations and drainages of splenic lesions, and biopsies of extrasplenic masses were excluded.
Examining the biopsy reports, written consent was gained for the procedure, and the WHO checklist was documented. 1% lidocaine was the choice of local anaesthetic, and every procedure was either performed by a radiology doctor (registrar or consultant) or a consultant ultrasonographer. 18-gauge (18G) core needle biopsy needles were used in all patients. Post-procedure care included 4-hour bed rest with regular clinical observations.
RESULTS
A total of 43 splenic biopsies were performed on 40 patients. Three patients had a prior splenic biopsy that either was negative and clinical concerns persisted for a malignant process, was of insufficient yield for further analysis, or was of necrotic material and therefore undiagnostic. There was documentation of a fourth patient who had a previous biopsy in a different region (London, UK), and therefore this was not included in the study.
There was some overlap in the requests for splenic biopsy with a number of requests querying both malignancy and infection. Twenty-two biopsies (51%) were performed to assess progression in the context of known or previous malignancy, 22 were performed to assess for malignancy in patients with no prior malignant history, and the remaining six biopsies (14%) were conducted to assess for infective lesions such as abscess. The spleen was biopsied because it was either the only site of abnormality and demonstrated suspicious characteristics, i.e., increasing in size on serial imaging, or proving that the biopsied lesion was a metastasis would have an impact on management, i.e., chemotherapy regimen, or histology was needed to guide treatment regimen. In one patient, the liver was previously biopsied but was not of sufficient quality, and therefore the spleen was biopsied.
Thirty of the 43 biopsies (71%) documented only one needle pass (coaxial needles were used in three of these), nine biopsies included two needle passes, one patient endured three passes, and one patient endured four passes, the latter of which was due to patient movement. For two of the biopsies, there was no documentation of the needle passes, they each documented two or three samples obtained.
Thirty-seven of the 43 samples (86%) proved to be diagnostic as there was sufficient sample yield for analysis to provide a diagnosis, as demonstrated in Table 2. Positive samples included both benign and malignant abnormal splenic tissue. Fourteen patients were diagnosed with lymphoma: diffuse large B cell lymphoma (DLBCL; n=9), Hodgkin’s lymphoma (n=4), and grey zone lymphoma (n=1). Two patients were diagnosed with leukaemia (n=2) and three patients were diagnosed with metastases from renal, endometrial, and oesophageal primaries. Benign conditions included non-specific reactive changes (n=5); infection (n=3), one of which was an Epstein-Barr virus (EBV) pseudotumour; granulomatous diseases such as sarcoidosis (n=2); and haemangioma (n=1). Figures 1–3 are CT and US images of a patient with DLBCL, sarcoidosis, and oesophageal metastasis, respectively. Tables 3 and 4 summarise the histological diagnoses of the positive biopsies.
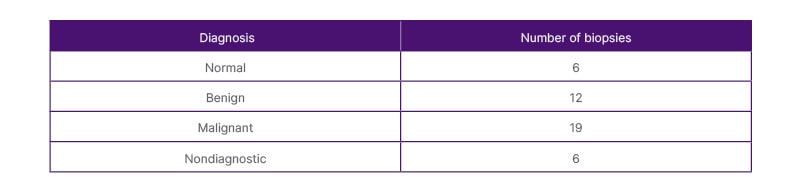
Table 2: Diagnosis of the ultrasound-guided splenic biopsies.
N/A: not applicable.
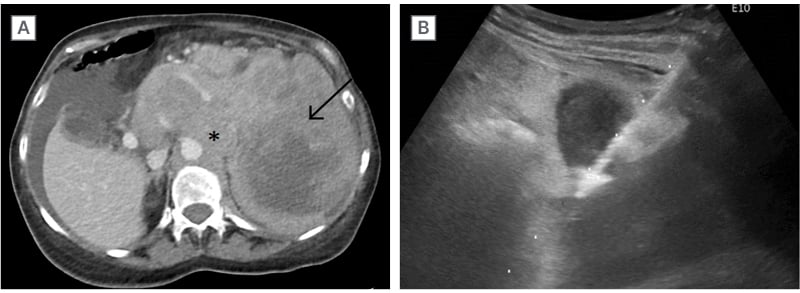
Figure 1: Diffuse large B cell lymphoma.
Patient presented with weight loss and early satiety. Contrast-enhanced CT Abdomen A) revealed a large heterogeneous splenic mass (arrow) and contiguous large volume lymphadenopathy (asterisk) in the upper abdomen. US-guided core needle biopsy of the spleen B) confirmed diffuse large B cell lymphoma.
US: ultrasound.
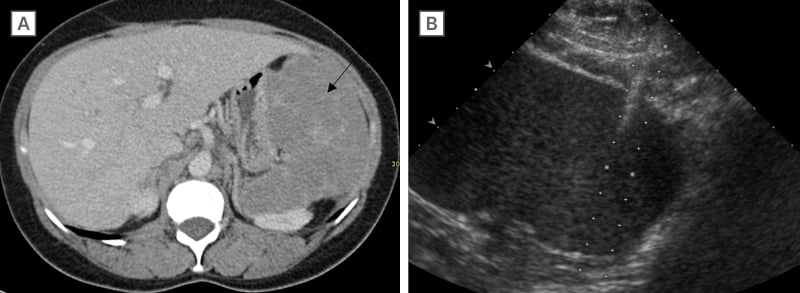
Figure 2: Sarcoidosis.
Contrast-enhanced CT abdomen A) was performed in this patient with palpable splenomegaly revealing a diffuse infiltrative enlarged spleen (arrow). US showed no focal splenic lesion, US-guided biopsy of the spleen. B) confirmed sarcoidosis.
US: ultrasound.
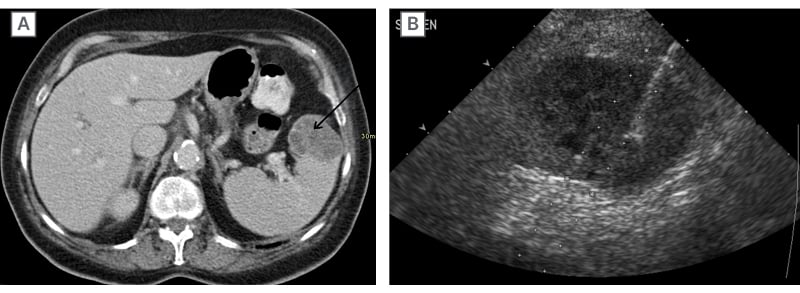
Figure 3: Oesophageal metastasis.
Contrast-enhanced CT abdomen A) in a patient newly diagnosed with oesophageal cancer demonstrated a solitary heterogeneous expansile mass in the spleen (arrow). US-guided core needle biopsy of this splenic mass B) revealed to be a metastasis (from the oesophageal primary).
US: ultrasound.
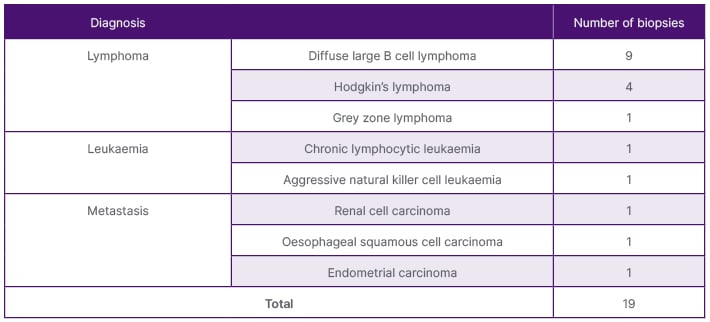
Table 3: Malignant histological diagnosis of the positive US-guided splenic biopsies.
US: ultrasound.
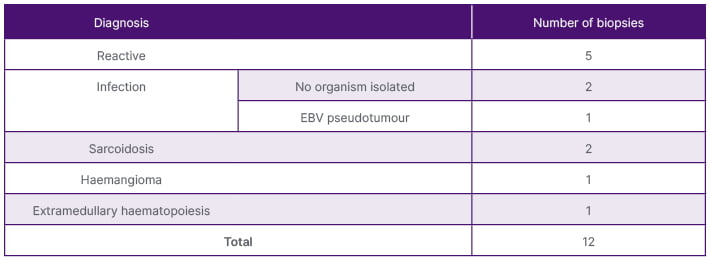
Table 4: Benign histological diagnosis of the positive US-guided splenic biopsies diagnosis.
EBV: Epstein-Barr virus; US: ultrasound.
After reviewing the patient’s medical records and follow-up imaging, the authors concluded that there were 30 true positives and zero false positives. There were five true negative biopsies, which meant that of the six histologically normal splenic tissue samples, five were truly normal. These results are summarised in Table 5. The one negative biopsy was proven to be false as the patient had a biopsy of an alternative site (clivus), which proved to be a diffuse large B cell lymphoma, with a histologically normal splenic biopsy. As a result, the overall diagnostic yield and accuracy were 86% and 84%, respectively. Excluding the repeat biopsies, the diagnostic yield was 93%.
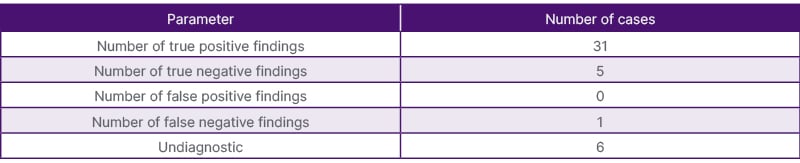
Table 5: Efficacy results of the US-guided splenic biopsies parameter number of cases.
US: ultrasound.
The remaining six of the 43 samples were undiagnostic as there was insufficient sample yield for analysis. Three of these undiagnostic biopsies were repeated, which revealed a DLBCL, infection, and normal splenic tissue. With regards to the other three undiagnostic splenic biopsies, the first patient proceeded to a liver biopsy which revealed epithelioid angiosarcoma (felt to be most likely from a splenic primary), the second patient received follow-up imaging to monitor the splenic lesion (revealed to be stable), and the third patient received an endoscopic US core needle biopsy that revealed a granulomatous disease.
After reviewing the electronic medical records, there were two minor complications (4.7%) where two patients developed small perisplenic haematomas. The first patient presented with hypotension and pain following the procedure, and therefore, had a triple-phase contrast-enhanced CT abdomen, which demonstrated a perisplenic haematoma with no evidence of active bleeding. Whereas the second patient developed tachycardia and a drop in haemoglobin and, interestingly, had an US of the abdomen, which also revealed a perisplenic haematoma with no further fluid or haematoma elsewhere in the abdomen. Both patients were conservatively managed with overnight admission. There were no major complications, although one patient died 1 month following the splenic biopsy, this was attributed to multi-organ failure secondary to underlying lymphoma rather than the biopsy procedure.
DISCUSSION
US-guided splenic biopsies are performed only when necessary due to the perceived risk of haemorrhagic complication. Splenic biopsy is a safer option compared to other alternatives such as a splenectomy which is associated with higher risk of morbidity (37%) and mortality (3%).3,4 Complications associated with splenectomy include thromboembolism and haemorrhage in the postoperative period, in addition to lengthier recovery times and hospital admission.3,4 On the contrary, in 41 of the 43 biopsy procedures performed, patients were discharged on the same day with no complication. Impaired immune response predisposing to a lifelong greater risk of infection and malignancy is the worrying long-term consequence of splenectomy.3,4 Percutaneous splenic biopsy allows the spleen to be preserved without the major complications associated with surgery.
In the authors’ study, the main indication of obtaining a splenic tissue sample was to assess for malignancy, either new malignancy or progression in the context of a known malignancy. On imaging alone, it is difficult to characterise a splenic lesion, particularly if it is a solid or a diffuse infiltrative lesion, and therefore, patients are referred for US-guided biopsy.5 Like any other organ, the spleen can be affected by many different disorders, such as malignancy, the most common of which are of haematological origin, such as lymphoma and leukaemia, as demonstrated in the authors’ study.6 Although it is rarely an organ to be infiltrated by metastases of non-haematological primaries, three of the authors’ samples demonstrated metastases from endometrial, oesophageal, and renal origin. Other lesions include benign tumours, infection, and granulomatous diseases such as sarcoidosis, as evidenced in the authors’ study.
The remainder six out of 43 samples produced insufficient yield for analysis. Most of the biopsy reports (70%) documented only one needle pass, and therefore, only one core tissue was obtained; this could have produced insufficient tissue for analysis. Increasing the number of needle passes increases the amount of tissue available for analysis, but this must be weighed against the risk of complication, particularly haemorrhage.Furthermore, malignant lesions can outgrow their vascular supply and develop necrotic centres that, if biopsied, will not provide useful information and render the biopsy nondiagnostic. The three splenic biopsies that were repeated revealed DLBCL, infection, and normal splenic tissue, and therefore, were valuable in repeating the biopsy. The other three patients endured a subsequent liver or endoscopic splenic biopsy or received follow-up imaging, with the latter demonstrating stability.
The authors’ study revealed two minor complications (4.7%) in which two patients developed small perisplenic haematomas. There were no major complications, specifically no large or active haemorrhage that would necessitate intervention. The authors did not include pain as a minor complication, as this is a common and expected symptom following a biopsy. Nonetheless, this has been classified as a minor complication in other studies, whether post-procedural pain is a true complication is subject to debate. The rate of overall complications in other studies ranged from 2–16.7%, major complications specifically occurred in up to 1.9% of all biopsies. Patel et al.1 reported a 2% minor complication rate, with only one of 52 cases developing a subcapsular haematoma requiring no intervention, using an 18G core needle.1 On the other side of the spectrum, Tam et al.5 reported a complication rate of 16.7% (1.9% major, 14.7% minor) using a 22G core needle. Major complications included splenic rupture and perisplenic haematoma requiring intervention, most of the minor complications were postprocedural pain.5 Olson et al.7 reported an 8.2% complication rate, encompassing a 7.2% rate for minor complications, including pain and perisplenic haematoma, and a 1% major complication rate, including haemothorax needing a chest drain and embolisation of an intercostal artery pseudoaneurysm. However, the study performed by Olson et al.7 included CT as well as US as an imaging modality aiding biopsy of the spleen, utilising 18G core needles. Sangiorgio et al.8 reported a 6.7% complication rate, which were all minor complications, with 16–20G core needle biopsies.
Overall diagnostic yield and accuracy were 86% and 84%, respectively, which are comparable to prior studies. For example, Tam et al.5 reported 92.3% and 84.7%, respectively; Patel et al.1 reported a diagnostic yield of 89.4%; and Olson et al.7 reported a diagnostic yield of 87.6% and a higher accuracy of 94.1%.1,2,7 These demonstrate the relatively high diagnostic yield and diagnostic accuracy of US-guided splenic biopsies.
The main limitation of the authors’ study was the sample size of 43 across 18 years; this is a relatively small sample number to extrapolate conclusions from but demonstrates how uncommon US-guided splenic biopsies are performed.
CONCLUSION
Despite other diagnostic methods, ultrasound-guided percutaneous splenic biopsy and histopathological examination remains the best diagnostic option in certain cases. The authors’ study at a tertiary centre has demonstrated it is a safe procedure with a high diagnostic yield and relatively high accuracy.







