Abstract
Introduction: The use of the caesarean section (C-section) in obstetric care has exponentially increased in the past few decades. The caesarean scar defect (CSD) is a potential complication of C-section and is associated with a wide range of problems. The purpose of this study was to compare the evaluation of the CSD in non-pregnant women by sonohysterography (SHG) and MRI.
Methods: This study was performed in patients having undergone a single C-section more than 6 months prior, presenting with abnormal uterine bleeding, dysmenorrhoea, or pelvic pain. Since ultrasonography and pelvic examination were inconclusive, these patients underwent MRI followed by saline infusion SHG. Measurements and characteristics of the ‘niche’ were acquired from both MRI and SHG and compared for analysis.
Results: Patients with a single C-section presenting with prolonged bleeding, spotting, and dysmenorrhoea were included in this prospective study. SHG and MRI were used to measure scar thickness, width, depth, and adjacent myometrial thickness, in which the findings concurred. The mean defect depth was greater in patients with postmenstrual bleeding.
Conclusion: SHG is noninferior to MRI, and SHG has the potential to assess the dynamic status of the CSD, with morphological clarity.
INTRODUCTION
The increase in the rate of the caesarean section (C-section) worldwide has raised concerns over the associated complications, such as the caesarean scar defect (CSD). The most alarming concern of the scar is its rupture during birth;1,2 apart from dramatic obstetric issues, for instance placenta accrete, gynaecological sequelae such as secondary infertility, pelvic pain, ectopic pregnancy, and abnormal uterine bleeding (AUB) are other entities reported to be associated with CSD.3 The potential for patients with these complications to undergo procedures such as uterine curettage, endometrial ablation, and hysteroscopy is only recently being explored.4 Evaluation of the CSD is performed by transvaginal sonography (TVS), saline infusion sonohysterography (SHG), hysteroscopy, and MRI; however, no consensus has been reached for the assessment gold standard.3-5 In this study, the authors compare the evaluation of the CSD in non-pregnant females by SHG and MRI, advocating for the ability of SHG to perform on par with MRI and for it to be utilised in low-resource settings.
MATERIALS AND METHODS
This study was conducted over a 2-year period in Srinagar, Jammu and Kashmir, India, on patients who had undergone a pelvic MRI for evaluation of symptoms such as AUB, dysmenorrhoea, and pelvic pain. It is pertinent to mention that these patients were referred for MRI after inconclusive ultrasonography, both transabdominal and transvaginal. The MRI was performed on the Siemens MAGNETOM Avanto 1.5T (Siemens Healthineers AG, Erlangen, Germany). T1 and T2-weighted images were acquired through standard planes. The sagittal T2-weighted scan of the uterus was examined for the presence of the caesarean scar. Only the cases with an identifiable scar on T2-weighted sagittal images were selected; these patients were educated on SHG and only those who agreed to the procedure were involved for further evaluation. Individuals excluded from the study were those who had undergone a C-section within the last 12 months, had undergone more than one C-section, were noncompliant, and those with possible contributory ancillary findings like fibroids, adenomyosis, and adnexal cysts, to exclude patient group variables. More than 300 pelvic MRI scans were evaluated and only 13 were selected for further assessment.
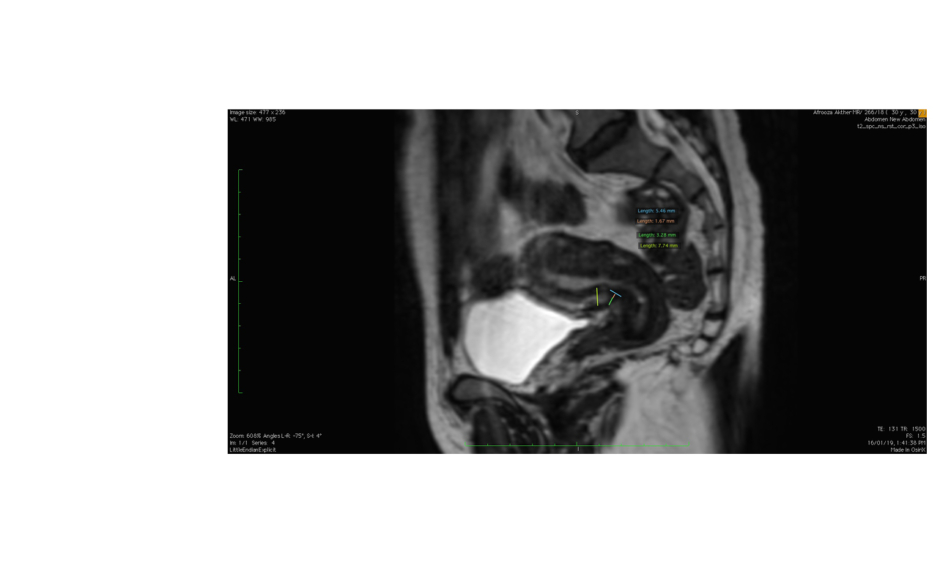
For the selected MRI examinations, the thickness of the scar was measured, i.e., the distance from the serosal surface of the uterus to the apex of the scar. The total myometrial thickness adjacent to the scar was also measured. In the case of a ‘niche’, the depth and width were measured (Figure 1).
Figure 1: Sagittal T2-weighted MRI image depicting the uterus with callipers marking the ‘niche’ and its measurements.
SHG was performed on Days 8–10 of the menstrual cycle, either in the same cycle or the next feasible cycle, with the exclusion of patients who were pregnant. The procedure was performed on the SonoSite M-Turbo® (Fujufilm, Bothell, Washington, USA). In the case of menorrhagia, SHG was performed (with informed consent) 2 days after cessation of bleeding by haemostatics. A Cusco’s speculum was introduced, with the patient in the lithotomy position and the cervix swabbed with a solution of saline and povidone-iodine. A Foley catheter, 8 French units in diameter, was introduced via the external os and the balloon inflated; this was followed by the instillation of 20–50 mL of sterile saline into the uterine cavity under sonographic guidance with focus on the uterine scar. The thickness of the residual myometrium, the thickness of the myometrium bordering the scar (the anterior myometrium), and the depth of the niche (a triangular, anechoic area at the presumed site of incision) were measured (Figure 2).
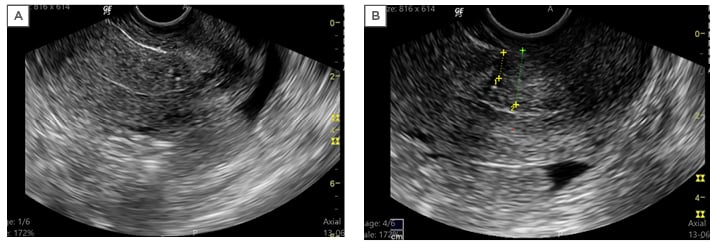
Figure 2: Imaging scans from a single patient. A) Transvaginal sonography showing the uterus in longitudinal section with thin endometrium and normal myometrium; B) saline hysterosalpingography showing the ‘niche’ with callipers marking the thickness of the residual myometrium (1) and adjacent myometrium (2).
RESULTS
The patient selection in this study allowed the authors to evaluate only those patients who had been symptomatic enough to have been referred for MRI. All the included patients had undergone a single C-section at least 12 months prior to the MRI.
Out of >300 pelvic MRI images, only 13 patients had measurable CSD on the T2-weighted sagittal MRI images (4.3%). Out of these, three had a history of two prior C-sections, one had coexisting multiple uterine fibroids, and one had adenomyosis; these patients were excluded from further study. The remaining eight patients had undergone a C-section more than 12 months prior. The mean age was 36 years. The most common symptom was prolonged cycles, defined as menstrual bleeding for >7 days (n=6) followed by postmenstrual spotting (a brown discharge at the end of the menstrual cycle) for at least 2 days (n=3). Other symptoms included dysmenorrhoea (n=5) and chronic pelvic pain (n=3). Emergency C-sections had been performed following prolonged labour (n=3) and for obstetric indications (n=4). None of the patients had previously given birth via vaginal delivery. Five patients had a history of gestational diabetes and an increased BMI of >28. Seven of these patients had anaemia during pregnancy. Five patients also reported having leukocytosis and fever postdelivery, which subsequently resolved.
On imaging, most of the patients had an anteverted uterus (n=6), while the others had a retroflexed uterus (n=3). The SHG and MRI findings correlated. An isthmocele was diagnosed when the CSD was at least 1 mm deep. The authors did not find any case with multiple defects. The mean defect width was 1 mm, while the mean defect depth was 3.4 mm with MRI and 3.6 mm with SHG. The mean scar thickness was 2.7 mm using MRI and 2.8 mm with SHG.
The mean myometrium adjacent to scar thickness ratio was 28% with MRI and 30% with SHG. There was no significant difference in the measurement results from the MRI versus SHG. The only difference was in the better delineation of the CSD on the SHG. All the defects had a triangular shape with both MRI and SHG.
The mean defect depth was greater in patients with postmenstrual bleeding, with a Spearman’s rank correlation coefficient of 0.87 (p<0.05). The rest of the correlations were not statistically significant (Figure 2A). On using the criteria of Osser et al.,6 who defined a large defect as a scar myometrial thickness of <2.2–2.5 mm with SHG, only two patients had large CSD, both of whom had postmenstrual spotting and dysmenorrhoea (Figure 3).
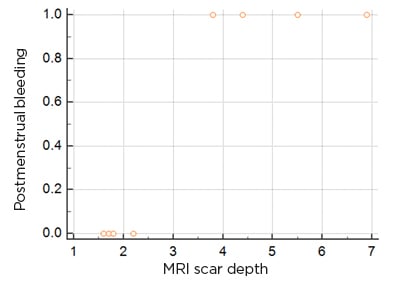
Figure 3: Scatter diagram of the scar depth in comparison to postmenstrual spotting.
DISCUSSION
When performed for the necessary reasons, the C-section, the most common global obstetric procedure, is a leading operation, having saved the lives of countless mothers and infants.5-10 In India, as per the District Level Household and Facility Survey-3, the C-section rate is 28.1% in private sector health facilities and 12% in public sector, much higher than the recommended 10% by the World Health Organization (WHO);7,11 this implies an increase in the incidence of CSD. It is reported that a rate higher than 10% may not provide further benefits but rather an increase in complications.2,7,10 Other complications reported (though rare) include uterine rupture and ectopic pregnancy with CSD.2,5-7,12 The prevalence of CSD have been reported with variations from 6.9–69.0%, and even up to 88.0%.2,5-7,13 Due to the fact that most patients are asymptomatic, it is likely that reported numbers represent only the tip of the iceberg.2,14
The niche, pouch, or isthmocele of a CSD is a normal tissue response due to scarring at the site of a previous scar defect.2,6,8–10,12,15-17 Its presence alone is not significant enough as it is commonly found after a C-section.14,18 It may take at least 6 months for the scar to heal, during which time the site will be oedematous; healing may continue for a few months after this.5,7 Additionally, vascular perfusion at the scar site may be affected by a range of factors including infection, diabetes, and nutritional status.7,9,16 For this reason, the authors chose to invoke the 12-month prerequisite in an attempt to evaluate after healing was complete.7
CSD has been reported to be associated with multiple gynaecological issues. In a cross-sectional study, 63.8% of women with a CSD had postmenstrual spotting and 31.0% of these women had dysmenorrhoea and 39.6% had chronic pelvic pain. Additionally, dyspareunia and higher rates of failure in intrauterine device placements have also been ascribed to a CSD.2,4,7,10
Thurmond et al.19 first reported abnormal bleeding with CSD by SHG evaluation. Postmenstrual spotting has been ascribed to have an independent relationship to the presence of a niche.13 Surgical isthmocele treatment has shown to improve fertility outcomes.2,16
It is hypothesised that menstrual blood collects in the scar subsequent to its poor contractility and then leaks out after the menstrual flow has stopped, presenting as spotting or prolonged bleeding.2,6,10,13,16,18 Morris et al.,20 on pathologic examination of the uterus in patients with AUB and a history of at least one C-section delivery, found widening of the lower uterine segment in 75%, overhang of congested endometrium above the scar recess in 61%, polyps within the scar in 16%, lymphocytic infiltration in 65%, residual suture material in 92%, capillary dilatation in 65%, fragmented endometrium in 37%, and scar adenomyosis in 28%.4 A chronic inflammatory environment can therefore contribute to AUB, chronic pelvic pain, and infertility.2,6,13 This warrants a further evaluation of the niche, preferably dynamic in the non-pregnant individual.
All of the defects found in this study were triangular in shape. In the literature, the most commonly reported shapes of the defect were triangular followed by round and oval.2,7,13,17 Prior vaginal deliveries do not seem to elevate the risk of a CSD.5,7 The ratio of the myometrial thickness at the scar to the thickness of adjacent myometrium indicates the degree of deficiency (>50%: severe deficiency).2,4,5 In this study, this ratio was 28% with MRI and 30% with SHG; hence, none of the defects were categorised as severe. Larger defects may inherently have contents and therefore can be picked up on plain TVS. The deeper the niche, the higher the risk for complications.2,15 Dehiscence extrapolates the occurrence of other complications such as scar pregnancy and placenta previa or accrete.2,18 Even so, such niches are more susceptible to injury during gynaecological procedures such as dilatation and intrauterine device implantation, making the documentation of these of prime importance.2
Although retroflexed uteri have been reported to have more common and larger CSD, the findings from this study are not comparable with only three patients having a retroflexed uterus.9,12 Obesity, prolonged pregnancy, peripartum infection, emergent C-sections, intraoperative complications, younger maternal age, prolonged duration of labour, advanced cervical dilatation, scar closer to the internal os, a retroflexed uterus, and a history of multiple C-section deliveries all increase the risk for a larger CSD.2,4,6,7,9 The authors found an increased BMI in five of the patients (62%). It has been stated that every additional unit of BMI increases the risk of an isthmocele by 6%.7 However, other studies have found no significant relationship between BMI and CSD.9 Prolonged labour was seen in three patients, all of whom were managed via emergency C-sections. Seven out of eight patients in the study had a history of an emergency C-section. Though prolonged labour itself is an independent risk factor for the development of an isthmocele, it is reported that the incidence of CSD is much higher in the emergency group rather than the elective group.7,9
Anaemia was recorded in seven out of eight patients. A reduced haemoglobin state with the requirement of blood transfusions has been previously implicated with scar dehiscence.6,9 The authors suggest the exploration of this association as anaemia was highly prevalent in the study set-up. The study also found a history of fever with leukocytosis postpartum in five patients. A possibility of infection can delay the healing of the wound and cause a future defect.6,9
TVS for the diagnosis of CSD has been reported since 1990 with an appearance of a wedge defect or a cystic mass between the bladder and lower uterine segment, which may be filled with debris.2,4,6,8,12,17 However, TVS can be misleading as it can both miss, as well as underestimate, the size of the defect.13,17,21 It is imperative to mention that in all the cases of this study, the niche had been missed on TVS. SHG has increased sensitivity and specificity for the detection of C-section scars by enhancing the defect and allowing its dynamic evaluation.2 A comparison between TVS and SHG for diagnosis of CSD found facilitation in delineating the borders of defects on SHG.4 Monteagudo et al.15 found that even though the site of the scar is identifiable by TVS, its depth and width could not be assessed without saline enhancement.13 Ofili-Yebovi et al.12 defined the degree of severity of the defect on ultrasound using the ratio of the myometrial thickness at the scar to the thickness of the adjacent myometrium, i.e., severe defect is a ratio of <50%.14 Though the first choice for screening, it seems plausible that symptomatic patients with negative TVS should invariably undergo SHG, as the defect can easily be missed with poor resolution or a low index of suspicion.14
MRI is not widely used as an investigative imaging tool for AUB, given its expense and low availability, especially in a low-resource setting.2,14 A novel study using contrast enhanced-MRI was able to detect larger pseudocavities in the anterior wall with saline, enabling clear contrast between fluid and muscular fibre during MRI and the visualisation of larger and clearer CSD margins.21 Wong et al.14 found a CSD prevalence of approximately 6.3% on MRI performed for other pathologies. Fiocchi et al.22 determined that the clinical value of 3T–magnetic resonance diffusion tensor imaging is better than ultrasound in predicting the thickness of the scar. The study also showed that the previous surgery in the anterior isthmus segment caused fibre disruption.22 This study also indicates that MRI and SHG perform equally with respect to the measurement of the defect and the myometrial morphology. Although MRI allows no definite advantage, SHG can evaluate the defect dynamically with evidence of any potential diverticulum formation. This study did not have any case of diverticulum, most likely due to the fact that only patients with a single C-section were included. This study may pave the way for future large-scale evaluation of the CSD by SHG as a potential cause for a spectrum of gynaecological issues.
This study is intrinsically limited by the small positive sample size and an implicated selection bias, as only symptomatic patients were received. However, the authors explored a twilight zone of scarce prospective studies on this topic. What is more, the fact that the study was undertaken in a low-resource setting in a developing country means that it takes a certain tenacity to get a patient and machine to coalesce for a diagnosis, along with convincing a patient to take an MRI, its cost factor, and the logistics of organisation in a government set-up.
CONCLUSION
The CSD warrants evaluation in the symptomatic non-pregnant uterus. Even though fashionably complex, MRI lags behind SHG in the potential to assess the dynamic status of the CSD, with morphological clarity paralleling that of MRI. The authors recommend the use of SHG as a first line of investigation in such patients, and also aim to invigorate larger prospective studies with a probable evolution of the investigation with advancing technology, e.g., to predict the probability of scar rupture to allow for consensus regarding the best treatment of these patients in order to reduce AUB, improve fertility, and avoid scar pregnancies and scar rupture.