Abstract
Postpartum haemorrhage (PPH) is a significant cause of maternal morbidity and mortality. The management includes a spectrum ranging from mechanical and pharmacological to surgical methods. The authors report a case of a 24-year-old primigravida who presented with complaints of abdominal pain, distension, fever, and diarrhoea a few days after her delivery. She had postpartum haemorrhage, for which compression sutures were applied. On radiological investigations, she was diagnosed with myometrial necrosis, following which she underwent a subtotal hysterectomy. Myometrial necrosis is a common complication encountered in the setting of postpartum haemorrhage secondary to compressive sutures. An early diagnosis and an appropriate management requires adequate radiological investigations.
Key Points
1. Myometrial necrosis is an expected complication with compression sutures for postpartum haemorrhage. The choice of compression sutures must be based on the clinical status of the patient, and the surgeon’s expertise.
2. Radiological features of myometrial necrosis include avascularity on colour Doppler and CT. Air foci might be encountered in the setting of infection.
3. Constant vigilance is necessary in patients who are high-risk for myometrial necrosis, in the form of clinical symptoms and appropriate radiological investigations.
INTRODUCTION
Postpartum haemorrhage (PPH) is a significant cause of maternal morbidity and mortality. PPH affects approximately 2% of all females who give birth. It is associated not only with nearly one-quarter of all maternal deaths globally, but is also the leading cause of maternal mortality in most low-income countries.1 PPH needs a rampant diagnosis, and adequate management. The management includes a spectrum ranging from mechanical and pharmacological to surgical methods. Physiological means of prevention of blood loss include the anatomical arrangement of the uterine muscular layer, which constricts the uterine vasculature during contraction;2 and the release of oxytocin during breastfeeding, which is a physiological uterotonic agent.3,4 Although the first-line management includes mechanical compression and the use of pharmacological agents, many patients require additional measures. Depending on the haemodynamic parameters, the patient may be offered compression sutures or uterine artery embolisation if vitally stable. If unstable, or if all other methods have failed to achieve haemostasis, they may be directly taken up for hysterectomy. Apart from managing the blood loss, it is equally important to watch out for complications associated with the measures adopted for haemostasis. One such dreaded complication is uterine myometrial necrosis, which requires hysterectomy as its management. Myometrial necrosis has been reported as a complication of embolisation and compression sutures for PPH, a sequelae to endometritis, and rarely post-Caesarean section.5,6 Clinically, it presents with abdominal pain, fever, diarrhoea, or vaginal discharge. The authors report a case of myometrial necrosis, following compression suture surgery for atonic PPH.
CLINICAL CASE
A 24-year-old primigravida (G1P0L0) was admitted to a secondary referral centre for safe confinement. After a trial of labour, she was taken up for emergency lower segment Caesarean section (LSCS) due to obstructed labour. LSCS was carried out under spinal anaesthesia. The baby was delivered headfirst, cried immediately after birth, and was handed over to the paediatrician. The placenta and membranes were expelled spontaneously and completely. The uterus was atonic, resulting in primary postpartum haemorrhage. The patient was administered uterotonic agents, failing which B-lynch compression sutures were applied with No. 1 Vicryl suture (Johnson & Johnson MedTech, New Brunswick, New Jersey, USA) for refractory PPH. Both the uterine arteries were ligated with absorbable sutures for controlling haemorrhage. The uterus was well contracted post-compression sutures, and haemostasis was confirmed. The abdomen was closed in layers. The patient was transfused with packed cell volume and fresh frozen plasma.
On postoperative Day 3, the patient developed five-to-six episodes of loose stools and vomiting, with gradually increasing abdominal distension. On postoperative Day 5, she developed occasional fever spikes, and was shifted to the ICU for paralytic ileus. CT on postoperative Day 7 showed dilated small bowel loops with bulky uterus, and few air foci in the uterine cavity. Ultrasonogram of the abdomen on postoperative Day 11 showed 100 cc of collection anterior and posterior to the uterus, with dilated small bowel loops. The patient developed daily fever spikes up to 102 oF, and was started on antibiotics.
The patient was referred to the Seth GS Medical College and KEM Hospital, Mumbai, India, for further management. On admission, the patient was afebrile and vitally stable, with soft and non-tender abdomen. On local examination, there was a wound gape of 2×1 cm (up to the muscular layer) with purulent discharge. Ultrasonogram of the abdomen showed a bulky uterus of size 17x15x9 cm with heterogenous echotexture and ill-defined endomyometrial junction, with multiple air foci in the endometrial cavity (Figure 1A), extending up to the scar site, suggestive of scar dehiscence. There was no vascularity in the myometrium on colour Doppler (Figure 1B). There was a heterogeneously hypoechoic collection of approximately 50 cc seen superior to the bladder, with no collection at the suture site. Contrast-enhanced CT scan of the abdomen showed enlarged postpartum uterus with multiple air foci in the endometrial cavity, with loss of endomyometrial junction and non-enhancing myometrium (Figures 2A and 2B). There was dehiscence in the anterior wall of the uterus at the LSCS site, with focal bulge contained by overlying serosa (Figure 2B). There was a collection measuring 4.4×2.6×2.3 cm in the pouch of Douglas.
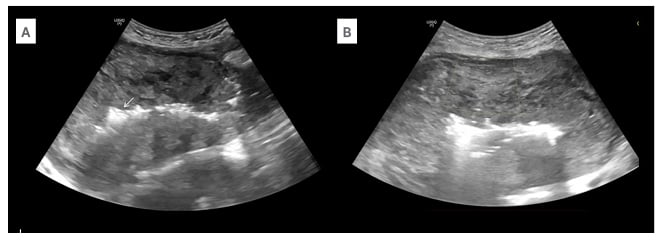
Figure 1: Ultrasound findings in myometrial necrosis.
A) Ultrasound of the abdomen in the longitudinal section shows a bulky postpartum uterus with heterogenous echotexture of the myometrium. There are air foci with dirty post-acoustic shadowing in the endometrium (arrow). B) Axial sections of the abdomen show no vascularity in the myometrium on colour Doppler with minimal scale.
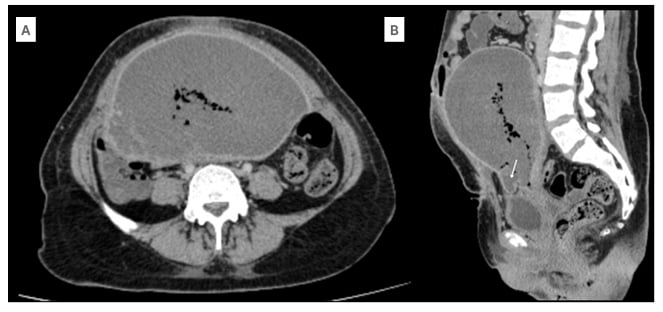
Figure 2: CT findings in myometrial necrosis.
A) Axial sections of the abdominal CT in the venous phase show lack of myometrial enhancement. The mean Hounsfield unit of the myometrium in post-contrast phase is 33. B) Sagittal section of the abdominal CT in the venous phase shows a bulge in the serosa at the scar site (arrow).
The patient underwent obstetric subtotal hysterectomy (Figure 3), in view of myometrial necrosis. After a stable course in the ward, the patient was discharged from the hospital.
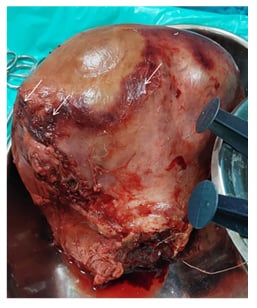
Figure 3: The specimen of the bulky postpartum uterus with areas of necrosis (arrows) post-subtotal hysterectomy.
DISCUSSION
The most common cause of PPH is uterine atony.7 Appropriate timely intervention is required to stem the haemorrhage. Physiological factors responsible for haemostasis following delivery might sometimes fall short, and might warrant the use of various pharmacological or surgical options. In the authors’ case, the bleeding could not be controlled with pharmacological agents, and the patient was subjected to uterine compression sutures and uterine artery ligation.
Various types of compression sutures commonly used for the management of PPH include B-Lynch sutures, Hayman sutures, and Cho square sutures.8-11 The B-Lynch technique was first introduced in 1997,12 and has since gained popularity as an effective means of achieving haemostasis in cases of atonic PPH. It involves the apposition of the anterior and the posterior uterine walls, with vertical sutures put around the uterus.13 B-Lynch, Hayman, and Pereira sutures are used to compress the uterine body. Cho square sutures are more useful in lower segment bleeding.14,15 Another variant of compression sutures is a simple U suture, which is easy to perform, and has lesser complications.8,9 Although these compression sutures are lifesaving and fertility-preserving, they do sometimes come with the cost of certain complications, including synechiae formation, haematometra, pyometra, and myometrial necrosis.16,17 Complications are more frequently encountered with Cho square sutures.14 The choice of sutures depends on the bleeding site, severity, disorders causing PPH, and the experience of the obstetric surgeon.14 Ligation of the uterine arteries and the internal iliac arteries may achieve adequate haemostasis, preserving fertility.18-20 The choice between uterine artery ligation and uterine compression sutures depends primarily on the preference of the surgeon. Both may be used as a first step in controlling bleeding refractory to medical management. Either of the methods may be used first, and if this fails, the other method can be implemented.21
The involution of the postpartum uterus ensures that the pressure created by the sutures is dynamic, and, as the uterus shrinks in size, the tension reduces. This, combined with the increased uterine vascularity and a rich anastomotic network, minimises the possibility of myometrial necrosis.22,23 The concomitant use of uterine compression sutures with devascularisation increases the risk of complications, compared to adopting the compression sutures alone.24,25 The setting of uterine necrosis can be explained by the lack of collateral supply to the uterus post-devascularisation. Incorrectly placed sutures can also pave the way for necrosis. Both arterial and venous infarction have been postulated as causes of myometrial necrosis. This was complicated by the presence of infection, as in the authors’ case. By the time the patient was referred to the authors’ centre, there was a significant delay, and a hysterectomy was done to prevent septicaemia, further morbidity, and mortality.
Radiology plays a vital role in diagnosing the cause, and also the complications encountered during the management of PPH. Ultrasound as the first line of imaging modality can diagnose retained products of conception as the cause of PPH, and can guide clinicians during curettage, ensuring adequate removal of products. Myometrial necrosis can be seen post-compression sutures, or post-uterine artery embolisation. It presents with complaints such as abdominal or pelvic pain, abdominal distension, fever, metrorrhagia, and vaginal discharge.26-28 It can be diagnosed on ultrasound, wherein the myometrium shows no internal vascularity on colour Doppler, as well as on CT, where the myometrium shows lack of enhancement in post-contrast sequences. Although rarely used, an MRI may also confirm the findings of myometrial necrosis. The necrosed myometrium shows intermediate-to-high signal intensity on T1-weighted images, and high signal intensity on T2-weighted images, along with a lack of early and delayed enhancement.29
Postoperatively, the patient should be under constant vigilance for myometrial vascularity. One must look for the signs and symptoms of necrosis as described above, and an ultrasound must be carried out at the earliest opportunity to detect the vascularity status.
CONCLUSION
Postpartum haemorrhage is a significant cause of mortality, and warrants rampant measures for its diagnosis and management. The choice of compression sutures should be tailored according to the status of the patient. Myometrial necrosis following compression sutures is a known complication, and early detection and appropriate management require adequate radiological investigations.