Meeting Summary
At the European Society for Medical Oncology (ESMO) Congress 2024 in Barcelona, Spain, the latest advancements in immunotherapy for colorectal cancer (CRC), gastro-oesophageal cancers, and hepatocellular carcinoma (HCC) were presented. Sara Lonardi from the Veneto Institute of Oncology, Italy, discussed the role of neoadjuvant immunotherapy in patients with high microsatellite instability (MSI-H) CRC, highlighting promising data from the CheckMate 8HW and NICHE-2 trials. Tania Fleitas Kanonnikoff from INCLIVA, Hospital Clínico Universitario de Valencia, Spain, provided insight into the use of immunotherapy-based regimens for gastro-oesophageal cancers, including treatment considerations based on key biomarkers and emerging treatment options. Thomas Decaens from the University of Grenoble-Alpes, France, presented results from several trials, including IMbrave150, HIMALAYA, and CheckMate 9DW, supporting the increasing role of immunotherapy combinations in first-line (1L) HCC treatment, which has been shown to improve overall survival in this challenging disease.
Colorectal Cancer: Novel Combination Therapies
Lonardi discussed recent advancements in immunotherapy for CRC, focusing on the impact of MSI-H status on treatment response. Historically, CRC was not considered responsive to immunotherapy until the identification of MSI-H, a marker of DNA mismatch repair associated with high mutation rates and neoantigen exposure.1-3 These insights have transformed the treatment landscape for MSI-H tumours, enabling the development of immune checkpoint inhibitors. Despite these advancements, MSI-H CRCs continue to exhibit complex immune evasion mechanisms, including overexpression of immune checkpoints such as programmed death-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), underscoring the ongoing need to explore combination therapies.4
Development and Results of Combination Regimens
The CheckMate 142 trial, a Phase II multi-cohort study, evaluated the combination of nivolumab (NIVO) and low-dose ipilimumab (IPI) in patients with MSI-H metastatic (m) CRC who had progressed after prior treatments. In a cohort of 119 patients treated with NIVO + IPI, the combination demonstrated a median progression-free survival (PFS) that had not yet been reached, with 52% of patients remaining progression-free at 5 years. Grade 3 or 4 treatment-related adverse events (TRAE) occurred in 32% of patients.5
Pembrolizumab was subsequently investigated in the 1L setting through the KEYNOTE-177 trial, comparing it with standard chemotherapy in patients with MSI-H/mismatch repair deficient (dMMR) mCRC. The trial enrolled 307 patients, with 153 receiving pembrolizumab and 154 receiving chemotherapy. The median PFS was 16.5 months (95% CI: 5.4–32.4) in the pembrolizumab group and 8.2 months (95% CI: 6.2–10.2) in the chemotherapy group (hazard ratio [HR]: 0.60; 95% CI: 0.45–0.79). Grade 3 or higher TRAEs were reported in 21.6% of patients in the pembrolizumab group compared to 66% in the chemotherapy group.6
Ongoing Trials and Future Directions
The randomised Phase III trial 8HW is currently comparing nivolumab monotherapy, nivolumab + ipilimumab, and standard chemotherapy in the 1L setting for MSI-H patients. This study features dual primary endpoints: PFS between NIVO + IPI and chemotherapy and a direct comparison between nivolumab monotherapy and nivolumab + ipilimumab. In the NIVO + IPI arm, which included 171 patients, the median PFS was not reached, with a CI of 38.4 months to not evaluable. This compared to a median PFS of 5.9 months (95% CI: 4.4–7.8) in the chemotherapy group of 84 patients (HR: 0.21; 97.91% CI: 0.13–0.35; p<0.0001). The study demonstrated that 79% of patients in the NIVO + IPI arm were progression-free at 12 months, and 72% remained progression-free at 24 months, compared to 21% and 14% in the chemotherapy group, respectively. The PFS benefit with NIVO + IPI was consistent across various analyses, including PFS by blinded independent central review. Data comparing nivolumab monotherapy versus nivolumab + ipilimumab are still awaited, and ongoing analysis aims to provide further insights. Additionally, NIVO + IPI had a different safety profile compared with chemotherapy, with fewer Grade 3/4 TRAEs, while improving health-related quality of life and reduced symptoms versus chemotherapy. Figure 1 demonstrates the data from CheckMate 8HW.7-9
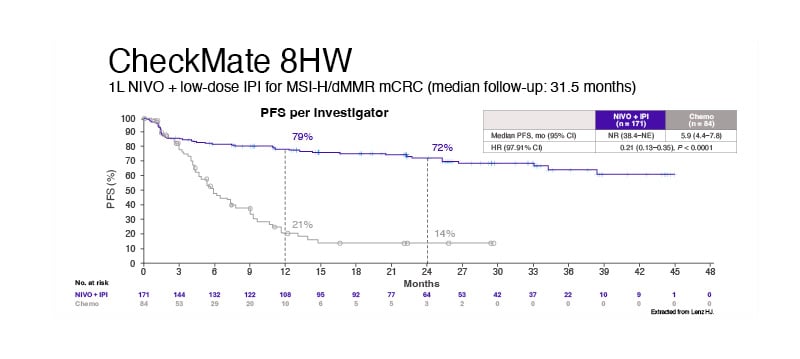
Figure 1: CheckMate 8HW study results.
1L: first line; dMMR: deficient DNA mistmatch repair; HR: hazard ratio; IPI: ipilimumab; mCRC: metastatic colorectal cancer; MSI-H: microsatellite instability high; NIVO: nivolumab; NR: not reached; PFS: progression-free survival.
Lonardi discussed the potential for further improving outcomes in MSI-H CRC through various dual immuno-oncology (IO) combinations. While multiple IO-based combinations are under investigation, she emphasised that using IO earlier in locally advanced resectable disease for MSI-H patients could deliver the most substantial improvement in outcomes.
Lonardi highlighted the NICHE-2 trial, which investigated neoadjuvant immunotherapy in patients with previously untreated, resectable clinical Stage II/III dMMR colon adenocarcinoma. In this trial, patients were treated with NIVO and low-dose IPI for one dose, followed 2 weeks later by a single dose of nivolumab monotherapy before surgery. A pathological complete response (pCR), defined as no residual viable tumour, was achieved in 67% of patients. Additionally, 95% of patients achieved a major pathological response, which includes both pCR and patients with ≤10% residual tumour. The trial also demonstrated promising long-term outcomes. With a median follow-up of 26.2 months, no disease recurrences were observed. The safety profile of this neoadjuvant IO approach was notable, with only 4% of patients experiencing Grade 3 or 4 immune-related AEs.10,11 Data presented at ESMO 2024 of 3-year disease-free survival from NICHE-2 showed unprecedented 3-year DFS of 100% in patients with high-risk, locally advanced dMMR colon cancer with only two cycles of neoadjuvant immunotherapy. All patients were circular tumour (ct)DNA negative at minimal residual disease time point, in line with 0% recurrences. Association of (early) clearance with pCR: ctDNA may aid in organ preservation.12
KRAS Mutations and Other Colorectal Cancer Subtypes
Lonardi closed the session by noting that only about 5% of CRC patients are MSI-H,13 highlighting the need to develop new strategies for the remaining 95% of patients with CRC. One promising approach is targeting Kirsten rat sarcoma virus (KRAS) mutations, specifically KRASG12C, which is present in approximately 3% of patients with CRC.14 Preclinical data suggest that activation of epidermal growth factor receptor signalling can overcome KRASG12C inhibition in CRC.15,16
The CodeBreaK 300 trial evaluated sotorasib + panitumumab versus standard of care (SOC) in pre-treated KRASG12C-mutant mCRC. The median PFS was 5.62 months in the higher-dose group (sotorasib 960 mg + panitumumab) and 3.91 months in the lower-dose group, compared to 2.20 months with SOC. The objective response rate (ORR) was 30% in the higher-dose group, 8% in the lower-dose group, and 2% with SOC. Grade 3 or higher TRAEs occurred in 36% of the higher-dose group, 30% of the lower-dose group, and 43% of the SOC group.17
The KRYSTAL-1 trial evaluated the efficacy of adagrasib combined with cetuximab in pre-treated KRASG12C-mutant mCRC. In this trial involving 94 patients, the median PFS was 6.9 months (95% CI: 5.7–7.4), and the median OS was 15.9 months (95% CI: 11.8–18.8). The ORR was 34%, with a median duration of response of 5.8 months. Grade 3 or 4 TRAEs were reported in 28% of patients. These findings highlight the potential of targeted therapies like adagrasib combined with cetuximab in managing KRASG12C-mutant mCRC.18
Lonardi concluded that MSI is the more robust biomarker in CRC and recommended testing all patients from the outset. Pembrolizumab and NIVO + IPI are the SOC in the 1L setting for MSI-H patients,10 and NIVO + IPI in the neoadjuvant setting has shown high efficacy in resectable MSI-H colon cancer, with a 68% pCR rate. Data presented at ESMO 2024 of 3-year disease-free survival from NICHE-2 showed unprecedented 3-year DFS of 100% in patients with high-risk, locally advanced dMMR colon cancer with only two cycles of neoadjuvant immunotherapy.11,12 Lastly, targeting KRASG12C and epidermal growth factor receptor is an effective therapeutic approach for advanced, pre-treated mCRC with a KRASG12C mutation. The continued exploration of these strategies will be essential in refining treatment options and optimising outcomes for patients with CRC.15,16
Gastro-oesophageal Cancers: The Role of Immunotherapy
Tania Fleitas Kanonnikoff
Overview of Gastro-oesophageal Cancers
Kanonnikoff provided an overview of oesophageal and gastric cancers, which rank as the 7th and 5th most common causes of cancer-related death worldwide.19 The main types of oesophagal cancer are oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC). Gastro-oesophageal adenocarcinoma (gastric, gastro-oesophageal junction, or oesophageal adenocarcinoma) is treated in a similar way. Gastric cancer is known to exhibit significant heterogeneity. Biomarkers such as human epidermal growth factor receptor 2 (HER2), MSI-H, programmed death ligand-1 combined positive score (PD-L1 CPS), and recently Claudine 18.2 are essential in guiding treatment.
ESMO Guidelines for 1L Treatment in Oesophageal Squamous Cell Carcinoma
The ESMO guidelines recommend platinum plus fluoropyrimidine chemotherapy in the 1L treatment for PD-L1-negative patients. For PD-L1-positive patients with a tumour proportion score (TPS) of ≥1%, NIVO + chemo or NIVO + IPI is advised. For patients with a CPS score of ≥10, pembrolizumab plus chemotherapy is recommended.20 As of July 2024, the Committee for Medicinal Products for Human Use (CHMP) recommended the approval of toripalimab + chemotherapy for the 1L treatment of advanced ESCC.21
CheckMate 648: NIVO + Chemotherapy Versus Chemotherapy in Oesophageal Squamous Cell Carcinoma
CheckMate 648 is a pivotal trial that evaluated NIVO + chemotherapy versus chemotherapy alone as a 1L treatment in ESCC. The trial included patients with varying levels of PD-L1 expression. The median OS for the entire population was 13.2 months (95% CI: 11.1–15.7) in the NIVO + chemotherapy group, compared to 10.7 months (95% CI: 9.4–12.1) in the chemotherapy-only group (HR: 0.77; 95% CI: 0.65–0.92). In patients with PD-L1 tumour cell expression ≥1%, also known as PD-L1 TPS, the median OS was 15.0 months (95% CI: 11.9–18.7) for the NIVO + chemotherapy arm versus 9.1 months (95% CI: 7.7–10.0) for chemotherapy alone (HR: 0.60; 95% CI: 0.47–0.77). In the subgroup of patients with tumour cell PD-L1 expression <1%, the HR for OS was 0.97, indicating that enriched benefit was observed in patients with higher PD-L1 expression.22
The long-term follow-up provided valuable insights into the survival benefit of immunotherapy combined with chemotherapy. Grade 3 or 4 TRAEs were reported in 49% of patients receiving NIVO + chemotherapy and 37% in the chemotherapy-only group.22
CheckMate 648: NIVO + IPI Versus Chemotherapy in Oesophageal Squamous Cell Carcinoma
An additional arm of CheckMate 648 assessed NIVO + IPI versus chemotherapy alone as a 1L treatment for ESCC. The trial reported a median OS of 12.7 months (95% CI: 11.3–15.5) for the NIVO + IPI group compared to 10.7 months (95% CI: 9.4–12.1) for the chemotherapy-only group (HR: 0.78; 95% CI: 0.65–0.92). In patients with tumour cell PD-L1 expression ≥1%, the median OS was 13.1 months (95% CI: 11.2–17.4) for NIVO + IPI versus 9.1 months (95% CI: 7.7–10.0) for chemotherapy (HR: 0.63; 95% CI: 0.49–0.81). For patients with tumour cell PD-L1 expression <1%, the HR for OS was 0.94, indicating that the benefit of NIVO + IPI was more pronounced in patients with higher PD-L1 expression. Grade 3 or 4 TRAEs were reported in 33% of the NIVO + IPI group and 37% in the chemotherapy-only group.22
KEYNOTE-590: PEMBRO + Chemotherapy Versus Chemotherapy in Oesophageal Squamous Cell Carcinoma
The KEYNOTE-590 trial evaluated PEMBRO + chemotherapy versus chemotherapy alone in ESCC. In the overall ESCC population, the median OS was 12.6 months (95% CI: 10.2–14.2) for PEMBRO + chemotherapy versus 9.8 months (95% CI: 8.6–11.1) for chemotherapy alone (HR: 0.71; 95% CI: 0.60–0.85).23
For ESCC patients with PD-L1 CPS ≥10, the median OS was 13.9 months (95% CI: 11.1–16.0) in the PEMBRO + chemotherapy group compared to 8.8 months (95% CI: 7.8–10.5) in the chemotherapy group (HR: 0.59; 95% CI: 0.45–0.76). The 5-year OS rate was 13.8% for PEMBRO + chemotherapy and 3.7% for chemotherapy alone, highlighting the long-term benefit of immunotherapy in this subgroup.24
Additionally, in patients with PD-L1 CPS <10, the OS HR was 0.84, indicating a less pronounced benefit in this lower expression group.24 Grade 3 or higher TRAEs were reported in 71.9% of patients receiving PEMBRO + chemotherapy and 67.6% in the chemotherapy-only group.
Fleitas discussed the duration of 1L therapies in her clinical practice. Current guidelines recommend chemotherapy combined with either PEMBRO or NIVO.19 In her practice, she typically discontinues oxaliplatin after eight cycles and continues with immunotherapy and fluoropyrimidine if there is a clinical benefit for the patient.
KEYNOTE-811 in HER2- Positive Gastric/Gastro-oesophageal Junction Cancer
For HER2-positive advanced gastric, gastro-oesophageal junction cancer, and EAC, the addition of PEMBRO to trastuzumab (TRAS) and chemotherapy is now a recommended treatment option for PD-L1 CPS ≥1, supported by results from the KEYNOTE-811 trial.24,25 The trial demonstrated significant improvements in PFS and OS when PEMBRO was added to the standard TRAS + chemotherapy regimen for the PD-L1 positive population.26,27
For patients with PD-L1 CPS ≥1, the median PFS was 10.9 months (95% CI: 8.5–12.5) in the PEMBRO group compared to 7.3 months (95% CI: 6.8–8.5) in the placebo group (HR: 0.71; 95% CI: 0.59–0.86). The median OS was 20.5 months in the PEMBRO group versus 15.6 months in the placebo group (HR: 0.79; 95% CI: 0.64–0.98). The ORR was 73.2% in patients with PD-L1 CPS ≥1 receiving PEMBRO + TRAS + chemotherapy, compared to 58.4% in the placebo group.26,27
Grade 3 or 4 TRAEs occurred in 58% of patients receiving PEMBRO and 50% of patients in the placebo group, reflecting the manageable safety profile of the combination therapy. This combination has become an established standard of care for HER2-positive patients with PD-L1 expression. Fleitas mentioned that in her practice she continues both pembrolizumab and trastuzumab until the disease progresses as a maintenance strategy.26,27
CheckMate 649 and KEYNOTE-859 in HER2-Negative Gastric/Gastro-oesophageal Junction Cancer/Oesophageal Adenocarcinoma
The CheckMate 649 and KEYNOTE-859 trials investigated the addition of immunotherapy to chemotherapy in advanced gastric, gastro-oesophageal junction cancer, and EAC, demonstrating that the addition of IO to chemotherapy showed superior benefit to chemotherapy alone. It also demonstrated that higher PD-L1 expression levels are associated with better treatment outcomes. EAC patients were only enrolled in CheckMate 649 study.
CheckMate 649 trial in HER2-negative gastric/gastro-oesophageal junction cancer/oesophageal adenocarcinoma28
- NIVO + chemotherapy was compared to chemotherapy alone.
- For PD-L1 CPS ≥5:
- Median OS: 14.4 months (95% CI: 13.1–16.2) with NIVO + chemotherapy.
- 11.1 months (95% CI: 10.1–12.1) in the chemotherapy group (HR: 0.70; 95% CI: 0.61–0.81).
- For PD-L1 CPS ≥1:
- Median OS: 13.8 months (95% CI: 12.4–14.8) with NIVO + chemotherapy.
- 11.4 months (95% CI: 10.7–12.3) in the chemotherapy group (HR: 0.75; 95% CI: 0.67–0.85).
- In all-randomised population:
- Median OS: 13.7 months (95% CI: 12.4–14.5) with NIVO + chemotherapy.
- 11.6 months (95% CI: 10.9–12.5) in the chemotherapy group (HR: 0.79; 95% CI: 0.71–0.88).
- For lower PD-L1 expression (CPS <1 and CPS <5), OS HRs were 0.98 and 0.95, respectively.
- Grade 3 or 4 TRAEs occurred in 60% of patients receiving NIVO + chemotherapy.
- 45% of patients experienced Grade 3 or 4 TRAEs in the chemotherapy- only group.
KEYNOTE-859 trial of pembrolizumab + chemotherapy versus chemotherapy in HER2-negative gastric/gastro-oesophageal junction cancer29,30
- PEMBRO + chemotherapy was compared to chemotherapy alone.
- For PD-L1 CPS ≥10:
- Median OS: 15.8 months (95% CI: 14.0–19.3) with PEMBRO + chemotherapy.
- 11.8 months (95% CI: 10.3–12.7) in the placebo group (HR: 0.64; 95% CI: 0.53–0.78).
- For PD-L1 CPS ≥1:
- Median OS: 13.0 months (95% CI: 11.6–14.2) with PEMBRO + chemotherapy.
- 11.4 months (95% CI: 10.5–12.0) in the placebo group (HR: 0.75; 95% CI: 0.66–0.85).
- Grade 3 or higher TRAEs were reported in 59.4% of patients in the PEMBRO + chemotherapy group.
- 51.3% of patients experienced Grade 3 or higher TRAEs in the placebo group.
Both trials highlight the critical role of PD-L1 expression as a predictive biomarker, with higher expression correlating with improved survival outcomes when immunotherapy is added to chemotherapy.
Fleitas emphasised the critical role of biomarker testing in the management of gastro-oesophageal cancers. Ongoing research and future trials will continue to refine these treatment strategies, with a focus on expanding therapeutic options and improving outcomes across different patient subgroups.
Hepatocellular Carcinoma: 1L Treatment Options in the Metastatic Setting
Thomas Decaens
Rationale for Immunotherapy in Hepatocellular Carcinoma
Decaens discussed immunotherapy’s role in HCC. HCC often arises from chronic inflammatory liver conditions such as non-alcoholic steatohepatitis, alcohol-related liver disease, and viral aetiologies, like hepatitis B and C infection.31-33 These chronic conditions create an immunosuppressive environment characterised by a low tumour mutation burden and fewer tumour-associated antigens, contributing to the resistance of HCC to immune-modulating therapies.34-36 The inherent immunotolerance of the liver further complicates effective immune engagement.37 In most cases in the West, cirrhosis is present, leading to modifications in liver endothelial cells that hinder tumour-associated antigen presentation.38 Additionally, the fibrotic liver alters cell trafficking, affecting the entry of immune cells from the blood.39
Mechanisms of Combination Therapy
Due to these challenges, combination therapy has become necessary in HCC treatment, as trials investigating single agents have often failed. In the current landscape, anti-PD-L1 agents, which enhance pre-existing T cell responses and cytokine production, are combined either with anti-vascular endothelial growth factor therapies to normalise tumour vasculature and reduce immunosuppression or anti-CTLA-4 therapies to increase T cell priming.40-42 This combination shifts a cold tumour to a hot tumour. Anti-CTLA-4 therapies, in addition to increasing T cell priming in the lymph node, enhance tumour antigen presentation and activate T cells, providing a broader immune response against the tumour.43,44
Clinical Trials and Efficacy of 1L Treatments
Decaens highlighted pivotal Phase III clinical trials comparing immunotherapy combinations to standard tyrosine kinase inhibitors (TKI), sorafenib (SOR), or lenvatinib in the 1L setting:
- IMbrave 150 Trial: This trial compared atezolizumab (ATEZO, PD-L1i) + bevacizumab (BEV, VGEFi) against SOR (TKI) in previously untreated patients with HCC. ATEZO + BEV demonstrated a median OS of 19.2 months compared to 13.4 months with SOR (HR: 0.66; 95% CI: 0.52–0.85; p<0.001). Grade 3 or 4 TRAEs were reported in 43% of patients receiving ATEZO + BEV and 46% of those receiving SOR. The rate of survival at 18 months was 52% in the immunotherapy group.45
- HIMALAYA Trial: This trial compared the combination of tremelimumab (TREME, CTLA-4) + durvalumab (DURVA, PD-L1I) versus SOR in a 1L setting. At a predetermined interim analysis, the study was positive, and the median OS was 16.4 months for the TREME + DURVA group compared to 13.8 months for SOR (HR: 0.78; 95% CI: 0.67–0.92; p=0.0037). The median follow-up of this trial was 48 months, and 25% of the patients survived at the end of the follow-up. The TREME + DURVA combination was associated with a lower incidence of serious TRAEs (17.5%) compared to the current SOC (ATEZO + BEV). The SOR group registered a cumulative serious TRAE rate of 9.4%.46 Serious AEs, regardless of attribution, occurred in 41.2%, 31.7%, and 29.7% of participants receiving STRIDE (tremelimumab + durvalumab), durvalumab, and sorafenib, respectively. Serious treatment-related AEs occurred in 17.5% of participants treated with STRIDE, 8.5% of participants treated with durvalumab, and 9.6% of participants treated with sorafenib. No new serious treatment-related AEs occurred after the primary analysis for STRIDE. The updated 5-year OS for STRIDE (tremelimumab + durvalumab) demonstrated a sustained OS benefit versus sorafenib, with OS rates of 19.6% versus 9.4% at 5 years and the OS rate ratios for STRIDE versus sorafenib increasing over time. There were no new serious treatment-related AEs after the primary analysis for STRIDE. This was presented at ESMO 2024.47
- CheckMate 9DW Trial: This recent trial evaluated NIVO (PD1i) + IPI (CTLA-4) versus lenvatinib (LENVA) or SOR. In 85% of the patients, the investigators chose lenvatinib, the strongest TKI in the market. This is a huge distinguishing factor compared to other studies in the 1L space, as competitors used SOR only. SOR is a first-generation TKI with a narrower efficacy profile than LENVA. NIVO + IPI showed a median OS of 23.7 months compared to 20.6 months for LENVA/SOR (HR: 0.79; 95% CI: 0.65–0.96; p=0.018). Grade 3 or 4 TRAEs were reported in 41% of patients in the NIVO + IPI group and 42% in the LENVA/SOR arm. The median follow-up was 35.2 months, which was relatively longer than the other trials, and in the NIVO + IPI arm, 38% of the patients were alive at the end of the follow-up.48
- CARES-310 Trial: The trial compared camrelizumab (CAMRE, PDL1-i)) + rivoceranib (RIVO, VGEFR-2i) versus SOR in the 1L setting for HCC. The combination of CAMRE + RIVO showed a median OS of 23.8 months compared to 15.2 months with SOR (HR: 0.64; 95% CI: 0.52–0.79; p<0.0001). The survival rates at 12 months were 76.6% for the CAMRE + RIVO group and 60.9% for the SOR group, highlighting the significant benefit of the combination therapy. Grade 3 or 4 TRAEs were reported in 81% of patients receiving CAMRE + RIVO and 52% in the SOR group.49,50 Table 151,52 refers to the first-line systemic therapy recommendations for first-line HCC. The results of the trial are summarised in Table 2.45-50
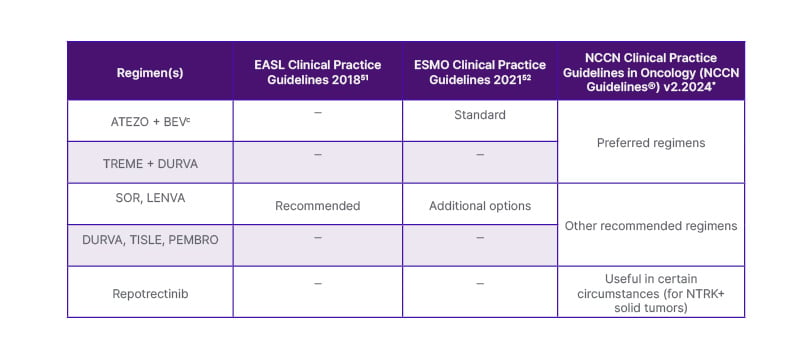
Table 1: First-line systemic therapy recommendations for advanced HCC.
*Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hepatocellular Carcinoma V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 10, 2024.
ATEZO: atezolizumab; BEV: bevacizumab; DURVA: durvalumab; EASL: European Association for the Study of the Liver; ESMO: European Society for Medical Oncology; HCC: hepatocellular carcinoma; LENVA: lenvatinib; NCCN: National Comprehensive Cancer Network; PEMBRO: pembrolizumab; SOR: sorafenib; TISLE: tislelizumab; TREME: tremelimumab.
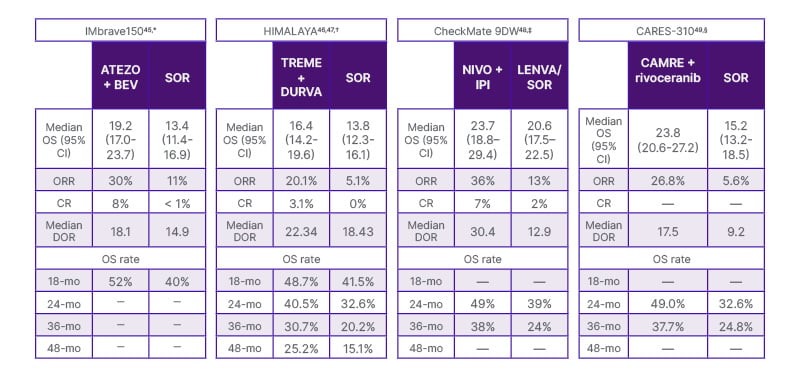
Table 2: Summary of immuno-oncology based 1L hepatocellular carcinoma treatments. Cross-trial comparisons should not be made due to differences in study design, patient populations, treatment interventions, and duration of follow-up, among others. Data are presented side-by-side for ease of viewing. Unless otherwise specified, all data are reported as mo.
* Median follow-up 15.6 mo.
† Median follow-up for median OS and 48-mo OS rate: –48 mo; median follow-up for other outcomes: –32 mo.
‡ Median follow-up: 35.2 mo.
§ Median follow-up: –18.5 mo.
1L: first line; ATEZO, atezolizumab; BEV: bevacizumab; CAMRE: camrelizumab; CR: complete response; DOR: duration of response; DURVA: durvalumab; I-O: immuno-oncology; IPI: ipilimumab; LENVA: lenvatinib; mo: month; NIVO: nivolumab; ORR: objective response rate; OS: overall survival; SOR: sorafenib; TREME: tremelimumab.
Clinical Considerations and Future Directions
Decaens discussed important clinical considerations, including the potential between increased exposure to CTLA-4 inhibitors and its assumed prolonged clinical benefit.50,53,54 Another consideration is that any patient with HCC is at risk of gastrointestinal bleeding due to the existing liver disease and the possibility of portal hypertension. The bleeding risk increases with advanced stages and antiangiogenic agents in combination regimens, as they could exacerbate portal hypertension.55-57
Looking ahead, Decaens highlighted the evolving landscape of HCC treatment. He noted that NIVO + IPI shows promise as a new SOC in 1L treatment. Researchers continue to explore new IO agents and combination therapies, with ongoing trials potentially defining a more optimised approach to managing advanced HCC. Decaens concluded that the recent advancements in 1L immunotherapy have significantly improved survival outcomes in HCC, offering a brighter outlook for patients with this challenging disease.







