Meeting Summary
This symposium took place during the European Society for Medical Oncology (ESMO) Congress 2021. Eric Van Cutsem welcomed attendees and provided an overview of the programme, which was designed to highlight the current treatment landscape in oesphageal cancer and explore future directions in cancer care. Elizabeth Smyth discussed the epidemiology of oesphageal cancer, the use of biomarker testing in patients with advanced disease to help guide treatment choices, and the current treatment paradigms for oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC). Lucjan Wyrwicz outlined the existing therapy options and the latest data on the treatment of metastatic oesophageal cancer in first- and second-line settings. He also discussed biomarkers and how they can be used to inform therapeutic decision-making in metastatic oesophageal cancer. Van Cutsem then explained how emerging immunotherapy regimens may potentially address the unmet needs of patients with oesophageal cancer. These could include the use of programmed cell death ligand 1 (PD-L1) inhibitors in earlier lines and earlier stages, and novel combination therapies involving an anti-PD-(L)1 agent. The session concluded with a live question and answer (Q&A) session exploring future directions.
Welcome and Programme Overview
Eric Van Cutsem
The symposium featured three talks and a live Q&A session. First, the current patient journey and standards of care in oesophageal cancer were outlined. This was followed by a discussion of the existing treatment landscape and the latest data for the treatment of metastatic oesophageal cancer in first- and second-line settings. Next, there was a presentation of biomarkers and how they inform therapeutic decision-making in metastatic oesophageal cancer. Finally, the Q&A session explored the unmet needs of patients with oesophageal cancer and how emerging immunotherapy regimens could potentially address these gaps.
The Patient Journey in Oesophageal Cancer: Standards of Care from Early-Stage to Metastatic Disease
Elizabeth Smyth
Oesophageal cancer is the sixth most common cause of cancer-related deaths worldwide.1,2 The two main histological subtypes of oesophageal cancer are OSCC and OAC.2 OSCC is the most common subtype of all oesophageal cancers worldwide.2 Diagnosis of both subtypes is often at a late stage when it is unsuitable for a curative treatment approach. As a result, the 5-year survival rate for oesophageal cancer is very low (19.9%).3 For patients with metastatic oesophageal cancer, options have previously been very limited, with just 5.2% of patients living for 5 years or more.3
OSCC is a tumour of the stratified squamous epithelium of the oesophagus. OSCC can occur anywhere in the oesophagus but most commonly affects the cervical, upper, and middle-thoracic oesophagus.4 Although the incidence of OSCC is relatively stable globally, in Western countries, such as the USA, Europe, and Australia, the incidence of OSCC has fallen.5 Although outcomes have improved for patients with oesophageal cancer overall, the prognosis for OSCC remains poor.6,7
OAC arises from the columnar glandular cells, which replace the squamous epithelium.6 OAC usually occurs in the lower third of the oesophagus.4 Tumours at the gastro-oesophageal junction (GEJ) are most commonly adenocarcinoma; squamous cancers rarely occur at the GEJ.8 Like OSCC, the incidence of OAC also varies by region and it is the most common subtype in the Western world, including North America, Western Europe, and Australia.2 In contrast to OSCC, the prognosis for patients with OAC has slightly improved over the past few decades, but survival is limited.9
Biomarker testing should be performed in patients with advanced oesophageal cancer to help guide treatment choices.10-12 The biomarkers used to select treatment for OAC are the same as those tested in gastric cancer: human epidermal growth factor receptor 2 (HER2), mismatch repair deficiency, high levels of microsatellite instability, and PD-L1. The latter should also be tested in OSCC.
The current treatment paradigm for oesophageal cancer can be broadly divided into OSCC and OAC. Starting with OSCC,13-17 Stage I can sometimes be treated with endoscopic resection, especially tumour 1, node 0 (T1N0). However, risk factors for recurrence may mean that these patients are not suitable for endoscopic resection and require surgical resection. Treatment for Stages II and III may or may not include surgery. This is because of the extreme sensitivity of OSCC to radiotherapy and the requirement for good cardiac and respiratory function. Those undergoing surgery should receive trimodality therapy, which refers to chemotherapy and radiotherapy followed by surgery. The chemotherapy and radiotherapy regimen is often based on the CROSS trial of weekly paclitaxel and carboplatin, although platinum and fluorouracil (5FU) might be used.18 A pathological complete response (pCR) can be expected in approximately 50% of OSCC treated with CROSS chemoradiotherapy (CRT) and surgery. However, for patients without pCR, the new standard of care after surgery is the PD-1 inhibitor nivolumab. Nivolumab after trimodality therapy in non-pCR patients doubles disease-free survival (DFS), although overall survival (OS) data have not yet been presented. The alternative treatment for patients with Stage II and III OSCC who do not wish to undergo surgery is definitive CRT. Given the high rates of pCR seen with CRT this is reasonable. Definitive CRT with planned salvage surgery has not been compared directly to trimodality therapy; this is being addressed in the NEEDS trial.19
If patients with OSCC recur locally after surgery or radiotherapy for locally advanced disease, further radiotherapy or salvage surgery may be an option. However, it is much more likely that these patients will follow a metastatic pathway. Standards of care for the first-line treatment of metastatic OSCC have changed this year, meaning that anti-PD-1 will become a standard of care for many patients, likely depending on PD-L1 status.
The recommended chemotherapy for advanced OSCC is platinum- and fluoropyrimidine-based. Pembrolizumab can be added to chemotherapy for OSCC patients in Europe with a PD-L1 combined positive score (CPS) >10 based on the KEYNOTE-590 results.20 It is very likely that there will be an approval for nivolumab in combination with chemotherapy based on the CheckMate 648 trial,21 but it remains to be seen whether that will be associated with PD-L1 status.
Finally, two approved PD-1 inhibitors are available in the second-line setting. Nivolumab can be used in a biomarker-unselected manner based on the results of the ATTRACTION-3 trial.22 Outside Europe, pembrolizumab is available for patients expressing PD-L1 with CPS >10. For patients previously treated with immunotherapy, second-line chemotherapy with a taxane or irinotecan is appropriate.
Moving on to OAC, there is some divergence from OSCC, both in early and late disease. Again, very early cancers might be suitable for endoscopic resection or surgery. There are two options for Stage II and III cancers. However, it should be noted that for resectable OAC in fit patients, surgery is curative while CRT is not. Before surgery, neoadjuvant radiotherapy can be considered; again, usually based on the CROSS trial data.18 Using CRT in OAC is not associated with the same levels of pCR seen in OSCC and is usually approximately 25%; however, this treatment does improve survival compared to surgery alone. Using data from KEYNOTE-577, it was recently established that nivolumab after surgery in patients who have not achieved pCR improves DFS.23 The alternative treatment for patients with OAC and GEJ adenocarcinoma that is operable is 5FU, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy based on the results of the FLOT4 trial.24 This is four cycles of chemotherapy before and after surgery. Neoadjuvant CRT and FLOT have not been formally compared and both are reasonable options for patients until results of the ESOPEC trial are available.25 After surgery for OAC, patients are much more likely to relapse systemically, rather than locally, compared to OSCC so salvage radiotherapy is used less often.
Once patients are in the metastatic part of the pathway, many of the initial treatments for OAC are very similar to OSCC. For example, the standard first-line chemotherapy is platinum- and 5FU-based. More than 20% of patients with oesophageal and junctional adenocarcinoma are HER2-positive and these patients should have trastuzumab added to their first-line chemotherapy to improve survival.
Over the past year or so, anti-PD-1 therapies have been added to first-line chemotherapy in clinical trials. This may or may not be based on PD-L1 testing. In Europe, pembrolizumab is approved for patients with PD-L1 CPS >10. The approval for nivolumab is as yet unknown but may be for CPS >5 based on the results of the CheckMate 649 trial.26
In second-line OAC, treatment differs from OSCC. Paclitaxel and the anti-angiogenic antibody ramucirumab are used, if available, or irinotecan is an alternative chemotherapy. Trifluridine/tipiracil is approved in Europe for third-line treatment of OAC of the GEJ.
In summary, there are a number of steps that can be taken to improve patient management in OSCC. The first goal is to improve survival, especially in patients with advanced cancer where this has historically been poor but is now improving.3 Second is to better understand the diagnostic and prognostic biomarkers in OSCC to inform clinical decisions.10 Third is to develop novel combination treatment strategies that are effective and well tolerated.27,28 The final goal is to implement effective strategies to address quality of life, given the toxicity profile of current treatments.29
Exploring the Current Treatment Landscape in Metastatic Oesophageal Cancer
Lucjan Wyrwicz
A number of trials have been published or are ongoing on the use of anti-PD-1 agents for the first- and second-line treatment of metastatic oesophageal cancer. Starting with first-line, pembrolizumab in combination with chemotherapy was approved in March in the USA for oesophageal cancer/GEJ that is not suitable for surgical resection or definitive chemoradiation.30 This combination was approved in June in the European Union (EU) for oesophageal cancer/HER2- GEJ (PD-L1 CPS ≥10).31 The combination of nivolumab with either chemotherapy or ipilimumab is under review.32
In the first-line setting, evidence for the use of pembrolizumab was obtained from the KEYNOTE-590 trial.33 The trial enrolled 749 patients 1:1 to pembrolizumab versus placebo, OACh on top of 5FU plus cisplatin. The key eligibility criteria were locally advanced unresectable or metastatic OAC or OSCC or advanced/metastatic GEJ or Siewert Type 1 adenocarcinoma; Eastern Cooperative Oncology Group performance score (ECOG PS) 0–1; and treatment-naïve. The primary endpoints were OS and progression-free survival (PFS). At data cut-off (2nd July 2020), the median follow-up was 10.8 months. The median overall survival was 12.4 months (95% confidence interval [CI]: 10.5–14.0) in the pembrolizumab group compared with 9.8 months (95% CI: 8.8–10.8) in the placebo group, with a hazard ratio (HR) of 0.73 (p<0.0001) (Figure 1). The median PFS was 6.3 months (95% CI: 6.2-–6.9) and 5.8 months (95% CI: 5.0–6.0) with pembrolizumab and placebo, respectively (HR: 0.65; p<0.0001) (Figure 2).
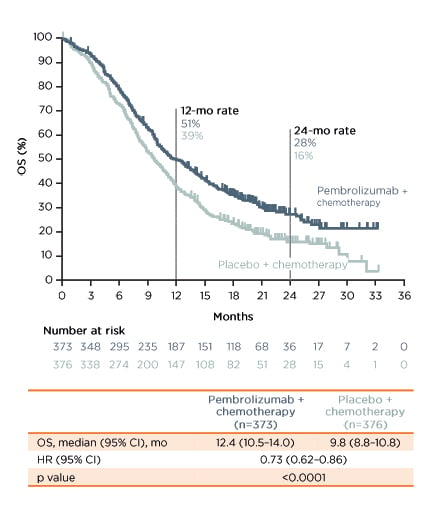
Figure 1: Overall survival in the KEYNOTE-590 trial.33
Investigator-assessed per RECIST v 1.1. Data cut-off: 2nd July 2020.
Pembrolizumab + chemotherapy led to a statistically significant improvement in OS compared to placebo + chemotherapy in the overall patient population and patients with oesophageal squamous cell carcinoma.
CI: confidence interval; HR: hazard ratio; mo: months; OS: overall survival.
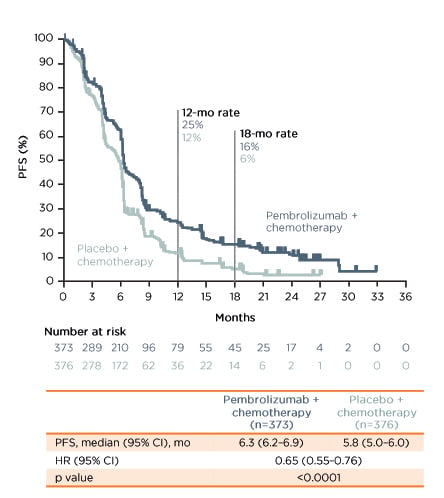
Figure 2: Progression-free survival in the KEYNOTE-590 trial.33
Investigator-assessed per RECIST v 1.1. Data cut-off: 2nd July 2020.
Pembrolizumab + chemotherapy led to a statistically significant improvement in PFS compared to placebo + chemotherapy in the overall patient population and patients with oesophageal squamous cell carcinoma.
CI: confidence interval; HR: hazard ratio; mo: months; OS: overall survival.
Data for nivolumab in the first-line was collected in CheckMate 648.21 A total of 970 patients were randomly allocated 1:1:1 to nivolumab plus chemotherapy (5FU and cisplatin) or nivolumab plus ipilimumab or chemotherapy (5FU and cisplatin). The key eligibility criteria were unresectable advanced, recurrent, or metastatic OSCC; ECOG PS 0–1; no prior systemic treatment for advanced disease; and measurable disease. The primary endpoints were OS and PFS in patients with tumour cell (TC) PD-L1 ≥1%, while OS and PFS in all randomised patients were secondary endpoints. At data cut-off (January 18, 2021), the minimum follow-up was 12.9 months. In all randomised patients, OS was superior with nivolumab plus chemotherapy versus chemotherapy alone in the intention-to-treat (ITT) population (13.2 versus 10.7 months; HR: 0.74; p=0.0021). However, in all randomised patients, the prespecified significance boundary for PFS per blinded independent central review was not met. Median PFS was 5.8 versus 5.6 months with nivolumab plus chemotherapy versus chemotherapy, respectively (HR: 0.81; 95% CI: 0.64–1.04; p=0.0355). Superior OS was also observed with nivolumab plus ipilimumab versus chemotherapy in all randomised patients (12.8 versus 10.7 months; HR: 0.78; p=0.0110). PFS was not hierarchically tested in all randomised patients as the primary endpoint (PFS in TC PD-L1 ≥1%) was not met.
ESCORT-1st examined camrelizumab in the first-line setting.34 The key eligibility criteria were histologically confirmed or cytologically confirmed OSCC, treatment naïve, advanced or metastatic disease, ≥1 measurable lesion per Response Evaluation Criteria in Solid Tumours (RECIST) 1.1, and ECOG PS 0–1. A total of 596 patients were randomised 1:1 to camrelizumab or placebo, both on top of chemotherapy. The co-primary endpoints were PFS per independent review committee and OS. First-line camrelizumab plus chemotherapy led to statistically significant improvement in OS and PFS compared to placebo plus chemotherapy. The median OS in the ITT population was 15.3 versus 12.0 months with camrelizumab and placebo, respectively (HR: 0.70; p=0.001). The corresponding values for median PFS were 6.9 versus 5.6 months (HR: 0.56; p<0.001).
Ongoing Phase III trials in the first-line setting include the RATIONALE 306 trial of tislelizumab,35 the ORIENT-15 trial of sintilimab,36 and a clinical trial of the anti-PD-1 agent HLX10.37
Moving to the second-line setting, pembrolizumab was approved in July 2019 in the USA for patients with ELCC and PD-L1 CPS >10.38 Nivolumab was approved in June 2020 in the USA39 and in November 2020 in the EU for patients with OSCC.40
Evidence for pembrolizumab in the second-line setting was obtained in the KEYNOTE-181 trial.41 The key eligibility criteria were confirmed OSCC or adenocarcinoma including HER2/neu-negative Siewert Type 1 GEJ adenocarcinoma; locally advanced, unresectable, or metastatic disease per RECIST v1.1; documented radiographic or clinical progression on ≥1 prior treatment; and ECOG PS 0–1. A total of 618 patients were randomly allocated 1:1 to pembrolizumab or chemotherapy. The three primary endpoints were OS in patients with PD-L1 CPS ≥10, OS in patients with OSCC, and OS in all patients. Regarding the results for all patients, there was an 11% reduction in the risk of death with pembrolizumab versus chemotherapy (median 7.1 versus 7.1 months; HR: 0.89; p=0.0560) (Figure 3). There was also an 11% increase in the risk of progression or death with pembrolizumab versus chemotherapy. The primary endpoint of OS was not met in patients with OSCC.
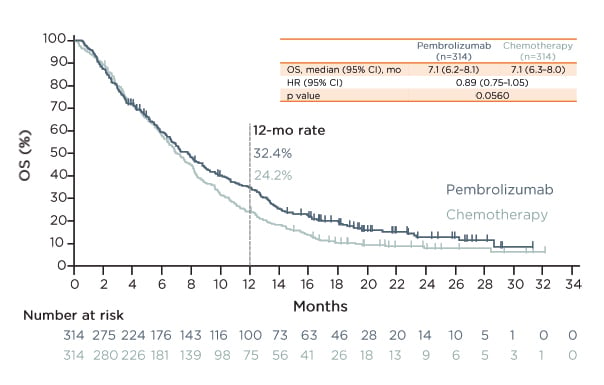
Figure 3: Overall survival in the KEYNOTE-181 trial.41
Data cut-off date: 15th October 2018.
In all patients, there was an 11% reduction in the risk of death and an 11% increase in the risk of progression or death with pembrolizumab versus chemotherapy.
CI: confidence interval; HR: hazard ratio; mo: months; OS: overall survival.
ATTRACTION-3 tested nivolumab in the second-line setting.22 In this trial, the key eligibility criteria were pathologically confirmed OSCC or OAC; refractory or intolerant to fluoropyrimidine-based and platinum-based chemotherapy; one prior treatment; one measurable or non-measurable lesion per RECIST v1.1; and ECOG PS 0–1. The trial randomised 419 patients 1:1 to nivolumab or chemotherapy and the primary endpoint was OS. There was a 21% reduction in the risk of death with nivolumab versus chemotherapy (median 10.9 versus 8.5 months; HR: 0.79; p=0.0264). There was also a 7% increase in the risk of progression or death with nivolumab versus chemotherapy (median PFS 1.7 versus 3.4 months; HR: 1.07).
RATIONALE 302 examined tislelizumab in second-line treatment.42 The inclusion criteria were advanced or metastatic OSCC, progression during or after first-line systemic treatment, and ECOG PS 0–1. A total of 512 patients were randomised 1:1 to tislelizumab or investigator-chosen chemotherapy. The primary endpoint was OS in all randomised patients. The trial demonstrated a 30% reduction in the risk of death with tislelizumab versus chemotherapy, with a median OS of 8.6 versus 6.3 months, respectively (HR: 0.70; p<0.0001).
Finally, ESCORT provided evidence for camrelizumab in second-line therapy.43 This trial enrolled patients aged 18–75 years with histologically or cytologically confirmed OSCC who had progressed on or were intolerant to first-line standard therapy, had ECOG PS 0–1, no known CNS metastases, and no prior PD-1 or PD-L1 therapy. A total of 457 patients were randomly allocated 1:1 to camrelizumab or investigator-chosen chemotherapy (docetaxel or irinotecan). The primary endpoint was OS. There was a 29% reduction in the risk of death with camrelizumab versus chemotherapy, with a median OS of 8.3 and 6.2 months, respectively (HR: 0.71; p=0.0010).
In summary, there are sufficient data to indicate that immunotherapy has an important role in the treatment landscape of both OSCC and OAC, using combination therapy in first-line and monotherapy in second-line. It should be noted that improved understanding of biomarkers will help to stratify and select the most suitable patients for therapy and monitor their clinical responses. The immune-related biomarkers currently being evaluated for immune checkpoint inhibitor therapy include tumour mutational burden,44 microsatellite instability,45 T-cell–inflamed gene-expression profile,46 and tumour-infiltrating lymphocytes.47,48 Tumour mutational burden, for example, has been associated with OS in studies of immune checkpoint inhibitors.44
Emerging Immunotherapy Treatment Strategies in Oesophageal Cancer
Eric Van Cutsem
There are a number of potential treatment strategies to address the unmet needs in oesophageal cancer. These include the use of anti-PD-(L)1 in earlier lines and earlier stages, and novel combination therapies involving an anti-PD-(L)1 agent.
Regarding earlier lines and earlier stages, CheckMate 64821 in OSCC and KEYNOTE-59033 in OSCC or OAC both showed that the addition of a PD-1 inhibitor (nivolumab and pembrolizumab, respectively) to first line-line treatment improved the outcome of patients with metastatic or advanced oesophageal cancer.
Many patients with early-stage resectable oesophageal cancer relapse after standard trimodality therapy. The challenge is therefore to move anti-PD-(L)1 agents into earlier lines in combination with trimodality therapy to increase pCR after neoadjuvant treatment, which associated with better outcome compared to non-pCR.49 Data are emerging on how this might be achieved. In CheckMate 577, patients were treated with CROSS CRT followed by surgery then randomised to nivolumab or placebo for 1 year.23 The trial showed that DFS was significantly longer with nivolumab compared to placebo.
A Phase II trial in OSCC of neoadjuvant tislelizumab compared with chemotherapy/CRT50 aims to capitalise on prior data indicating that immunotherapy agents may work synergistically with chemotherapy or CRT in the pre-operative setting. The ongoing Phase III KEYNOTE-585 study in patients with gastric/GEJ cancer is investigating the addition of pembrolizumab to standard perioperative chemotherapy.51
A series of trials are being conducted in patients with inoperable, locally advanced oesophageal cancer. Locoregional recurrence-free survival in patients with oesophageal cancer after definitive CRT remains frequent.52
On the other hand, radiotherapy and chemotherapy can exert immunomodulatory effects; therefore, combination with agents targeting the PD-1/PD-L1 axis may result in synergistic treatment responses. For example, treatment with anti-PD-1 therapy after chemotherapy and/or radiotherapy has an effect on tumour cells.53 Trials are being designed and are ongoing examining the combination of anti-PD-(L)1 agents with CRT in patients with inoperable OSCC.27 For example, the RATIONALE 311 trial is investigating the combination of tislelizumab and CRT in inoperable OSCC on PFS.54 Another Phase III trial in OSCC is examining the effect of camrelizumab plus definitive CRT on PFS.55 The KUNLUN Phase III trial is also testing the addition of an anti-PD-(L)1 agent to definitive CRT on PFS in OSCC, in this case with durvalumab.56 Finally, the Phase III KEYNOTE-975 is evaluating the addition of pembrolizumab to definitive CRT in patients with oesophageal/GEJ cancer.57
Several factors should be considered regarding the use of anti-PD-(L)1 agents in earlier lines and stages of oesophageal cancer. It will be important to identify biomarkers for optimal clinical outcomes, to examine the appropriate strategy after first-line combination therapy of anti-PD-1 plus chemotherapy, and to select both the optimal anti-PD-(L)1 regimen and dose of radiation. The safety profile of treatment with CRT and anti-PD-(L)1 agents in oesophageal cancer must be determined. Despite these unanswered questions, there is a clear rationale for continuing to develop these strategies.
Turning to novel combination therapies involving an anti-PD-(L)1 agent, OACh step of the anti-tumour immunity pathway provides a potential opportunity, such as immunotherapy plus radiotherapy/chemotherapy, targeted therapy, cell therapy, or additional immunotherapy.58,59 Ongoing studies of anti-PD-(L)1 combined with anti-T-cell Ig and ITIM domain (TIGIT) include the Phase II AdvanTIG-203 study, which is examining tislelizumab plus ociperlimab versus tislelizumab plus placebo for the second-line treatment of patients with advanced or metastatic OSCC with PD-L1 visually estimated CPS ≥10.60 In the first-line setting, the Phase III SKYSCRAPER-08 trial is testing atezolizumab combined with tiragolumab and chemotherapy versus chemotherapy alone in patients with unresectable locally advanced or metastatic OSCC.61
Clinical biomarkers are also needed to identify patients who may derive the most clinical benefit from novel combination therapies with anti-PD-(L)1 agents. In addition, the optimal combination regimen and treatment sequence will need to be determined. More data are required on the safety and tolerability of novel regimens.
In summary, unmet needs in locally advanced disease may be addressed by the addition of anti-PD-(L)1 agents upfront and the use of systemic therapy over surgical approaches, while in metastatic disease unmet needs may be addressed with novel combinations and chemotherapy-free options.62,63
Live Q&A
Eric Van Cutsem, Elizabeth Smyth, and Lucjan Wyrwicz
The session concluded with a live Q&A session about the future directions of immunotherapy in patients with oesophageal cancer. The panellists agreed that there is evidence that immune checkpoint inhibitors are effective with a number of different chemotherapy backbones. This means that physicians have a choice of backbone therapy.
Moving on to biomarkers, Smyth recommended PD-L1 testing in patients with OSCC and using pembrolizumab plus chemotherapy in patients who express PD-L1 with CPS >10 in first-line. While second-line is biomarker agnostic, Van Cutsem noted that there is some evidence that pembrolizumab is more effective in high expressers of PD-L1, but negative patients do show some response in this setting. This will become challenging in the future with checkpoint inhibitors moving first-line. Wyrwicz predicted that in 2 years the strategy will likely be similar to lung cancer where early immunotherapy is the focus for high PD-L1 expressers, while for low expressers immunotherapy is reserved for the refractory setting after chemotherapy. In addition, Smyth noted that very large cross-platform, cross-antibody validation studies are needed, as were performed in lung cancer.
Smyth highlighted claudin 18.2 as an attractive marker because it is expressed only on the tumour, thereby reducing off-target effects. The Phase II FAST study of zolbetuximab, an anti-claudin 18.2 antibody, showed positive results in terms of PFS and OS64 and the Phase III Spotlight trial is currently recruiting.65 More data are needed regarding co-expression of claudin 18.2 and immune checkpoint molecules like PD-1 and whether that will be impacted in future in combination therapy.
Van Cutsem point to another challenge, which is examining a strategy of PD-1 antibodies plus cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies in patients pre-treated in first-line with chemotherapy versus PD-1 antibodies. While many trials are still needed, the good news is that a lot of new data are coming through in both OSCC and OAC.








