Speakers: Eric Van Cutsem,1 Angela Lamarca,2 Nicola Normanno,3 Arndt Vogel4
1. University Hospitals Gasthuisberg Leuven and KU Leven, Belgium
2. The Christie NHS Foundation Trust and University of Manchester, UK
3. Fondazione IRCCS Istituto Nazionale dei Tumori, Naples, Italy
4. Hannover Medical School, Hannover, Germany
Disclosure: Van Cutsem is on advisory boards for Array, Astellas, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Daiichi, Halozyme, GlaxoSmithKline (GSK), Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Pierre Fabre, Roche, Servier, Sirtex, and Taiho; has received research grants from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Celgene, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier; and has received consultancy fees from Incyte. Lamarca has received honoraria or consultation fees from Eisai, Nutricia, Ipsen, Roche, and QED; participated in company sponsored speaker bureau for Pfizer, Ipsen, Merck, Incyte, and QED; and received travel and education funding from Ipsen, Pfizer, Bayer, AAA Pharmaceutical, Sirtex, Delcath, Novartis, Mylan, Celgene, Abbott, and Bayer. Normanno has received speaker’s fees and participated in advisory boards with MSD, Qiagen, Bayer, Biocartis, Illumina, Incyte, Roche, BMS, Merck, Thermo Fisher, Boehringer Ingelheim, AstraZeneca, Sanofi, and Eli Lilly; research grants from Merck, Sysmex, Thermo Fisher, Qiagen, Roche, AstraZeneca, Biocartis, and Illumina; and is President of the International Quality Network for Pathology (IQN Path) and of the Italian Cancer Society. Vogel has received speaker, consultancy, and advisory role fees from Amgen, Roche, Bayer, Sanofi, BMS, Lilly, Novartis, EISAI, AstraZeneca, Merck, Incyte, Ipsen, Pierre Fabre, MSD, Sirtex, BTG, Servier, and Terumo; research funding from Servier; and is a commercial medical education provider with OncLive.
Acknowledgements: We thank Dr Allison Kirsop, Scientific Writers Ltd., Edinburgh, UK for manuscript writing services.
Support: This article was funded by Incyte Biosciences International.
Citation: EMJ Oncol. 2021:9[6]:2-10.
Meeting Summary
Eric Van Cutsem opened the symposium with a reminder of the progress that has been made in the treatment of bile duct cancers also called cholangiocarcinoma (CCA), and the session covered aspects of better patient identification, biomarkers, genomic testing, and emerging therapies. The speakers discussed the positive gains made from the evolving knowledge base amassed in recent years, and also the challenges associated with precision medicine.
![]()
Corrigendum: Moving Towards Precision Medicine for Patients with Cholangiocarcinoma
Speakers: Eric Van Cutsem, Angela Lamarca, Nicola Normanno, Arndt Vogel
Original citation: EMJ Oncol. 2021;9[6]:2-10.
Date correction published: 27.10.21
In the symposium review article in EMJ Oncology 9[6] 2021 (pages 2–10) the following sentence is incorrect “However, some 15% of gene fusions in iCCA are intrachromosomal, occurring within the same chromosome as the FGFR fusion is located. Consequently, if the FGFR2 rearrangement is small, the FISH analysis may provide a false negative result.” The sentence should read “However, some 50% of gene fusions in iCCA are intrachromosomal, occurring within the same chromosome as the FGFR fusion is located. Consequently, if the FGFR2 rearrangement is small, the FISH analysis may provide a false negative result.”
EMJ apologises for the error and any inconvenience caused.
![]()
Addressing the Burden of Cholangiocarcinoma: How Can We Better Identify Patients?
Eric Van Cutsem
Current diagnostic approaches in CCA are inadequate, without additional histological confirmation and, with no efficient screening strategy, identifying the patient population at risk remains problematic.1,2 Epidemiological data on the global incidence and mortality of this aggressive cancer revealed an increase over the last few decades,3 with significantly variable rates in different regions of the world.4,5 While mortality trends from intrahepatic (iCCA) and extrahepatic (eCCA) neoplasms may be inconsistent, a general increase has been observed over the last 15–20 years.1,3
Approximately 70% of patients have advanced disease at diagnosis, as many are asymptomatic in the early stages, or may present with non-specific symptoms.1 It is difficult to differentiate iCCA from hepatocellular carcinoma or from metastatic disease of other cancers, and pathology expertise is needed to fully examine patient biopsies.1 Biopsies for histological diagnosis need to be of high-quality to enable accurate diagnosis, and, importantly, for molecular profiling, which is recommended in patients with advanced CCA.6,7
On presenting with advanced CCA, the prognosis for patients is poor, with limited treatment options and a rapidly declining quality of life.1,8,9 If patient outcomes are to improve, multidisciplinary expert teams are needed to provide a more comprehensive understanding of an individual’s disease biology. Optimal treatment options can then be determined for a patient’s specific needs.10
The Importance of Biomarkers in Cholangiocarcinoma
Angela Lamarca
The importance of biomarkers in CCA has gained significance more recently as targeted therapies such as fibroblast growth factor receptor (FGFR), isocitrate dehydrogenase (IDH), and TRF (TERF-1 gene) inhibitors have become part of a patient’s treatment strategy, in addition to first-line systemic chemotherapy and second-line folinic acid therapy.1 As one of several rare cancers, Lamarca explained that the incidence rate of CCA, particularly iCCA, is rapidly increasing, stating that “these are not going to be rare cancers forever.” The prognosis for iCCA is very poor with a 5-year overall survival (OS) rate of <20% due to late-stage diagnosis, and there is a high relapse rate.4,11,12 Estimated future incidence projections show that pancreatic cancer, and liver and intrahepatic bile duct cancer will be the second and third most common causes of cancer-related mortality, respectively, by 2040.13
To date, patient selection criteria for clinical trials of targeted therapies has not been dependent upon specific biomarkers, and these trials have not provided the anticipated results. Today, multiple CCA biological pathways are now better understood,14 providing identifiable, targetable alterations for tailored treatments according to specific biomarkers. This progress has enabled a precision medicine approach for patients with CCA, and individualised therapy is now a reality for many.
Generally, mutations across biliary tract cancers (BTC) are highly variable with differences identified between iCCA and eCCA, as well as in mutations associated with gallbladder cancer. The main identified targetable alterations in iCCA are IDH1 and FGFR, which are the most understood targets to date, whereas in eCCA these targets are HER2 pathway alterations, for which more data are needed (Figure 1).15 When selecting patients for immunotherapy, Lamarca explained how tumour agnostic approaches are also important, e.g., NTRK fusions or mismatch repair deficiency, as there may be treatments available for patients beyond the established CCA therapies. Focusing on specific biomarkers of BTC, the most frequently found alterations are for iCCA; approximately 15–20% of patients show IDH1 mutations and FGFR2 fusions.15 Although they do exist, BRAF mutations are rarer and, when found, they can be used to offer patients a specific treatment strategy.
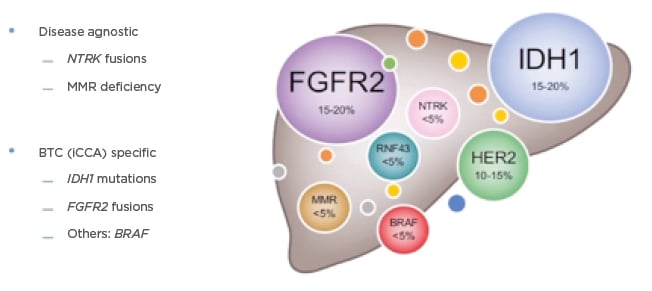
Figure 1: Current overview of ‘precision medicine’ in biliary tract cancers.
BTC: biliary tract cancer; FGFR: fibroblast growth factor receptor; iCCA: intrahepatic cholangiocarcinoma; IDH: isocitrate dehydrogenase; MMR: mismatch repair; RNF43: ring finger protein 43.
Adapted from Lamarca et al., 2020.15
IDH1 Inhibitors
Between 10–20% of iCCA cases are IDH1 mutant and with this mutation, there is a gain-of-function enzyme activity promoting tumourigenesis.14 IDH1 inhibitors produce an anti-cancer effect and the randomised Phase III clinical trial of ivosidenib (AG-120),16 a first-in-class, small-molecule targeted inhibitor of mutated IDH1, revealed progression-free survival (PFS) was significantly improved and well tolerated by patients in the treatment arm compared with those in the placebo group. The median PFS rate for ivosidenib was 2.7 months versus 1.4 months for placebo (hazard ratio: 0.37). At 6 months, 32% of patients in the treatment arm were progression-free compared with 0% for the placebo group, and at 12 months the PFS rate was 22% versus 0%, respectively. The median OS adjusted for cross-over of the placebo group to the ivosidenib arm was 10.8 versus 6.0 months with placebo (9.7 months unadjusted). These findings demonstrate the clinical benefit of targeting IDH1 mutations in advanced, IDH1-mutant CCA, and consequently there are patients who would clearly benefit from this treatment.16
Ivosidenib in Biliary Tract Cancers: Safety Data
As was found with the FGFR2 inhibitors, most side effects were Grade 1 or 2, indicating a clear benefit of these targeted therapies. Treatment-emergent adverse events were similar to those found for FGFR2 inhibitors but were considered manageable and do not prevent patients with CCA from receiving treatment.16
FGFR2 Inhibitors
Research investigations of FGFR2 mutations have, unfortunately, not produced particularly promising results; the highest activity has been found with FGFR2 fusions identified in approximately 10–20% of iCCA cases.15 FGFR2 receptors activate cell proliferation, migration, differentiation, and survival, and by inhibiting these receptors an anticancer effect may follow. Currently, there are several FGFR2 inhibitors in the development stage; infigratinib,17 pemigatinib,18 futibatinib,19 and derazantinib.20 All four inhibitors have been developed in patients with pretreated CCA, and the majority were iCCA cases. Pemigatinib was designated an orphan medicine by the European Medicines Agency (EMA) in August 2018,21 and U.S. Food and Drug Administration (FDA)-accelerated approval was granted to pemigatinib in April 2020,22 and to infigratinib in May 2021.23 Results from these four Phase II trials revealed a high response rate, considering that the cohort comprised heavily pretreated patients. In patients who did not reach a partial response (PR), a reduction in marker lesions was still achieved, which is an unusual and positive result for this patient population. However, to date, there are no head-to-head studies, which would enable cross-study comparisons. Moving forward, the role of these drugs will be explored in the first-line setting and also at an earlier stage in the disease pathway for patients with CCA.24,25 New strategies where FGFR2 inhibitors could be combined with chemotherapy still require clarification, and more research is needed to investigate the mechanisms of primary and secondary resistance in patients.
The importance of tumour agnostic biomarkers cannot be overlooked. Fewer than 5% of patients with BTC have BRAF gene mutations, yet these mutations can be targeted for treatment. Results of a Phase II, open-label, single-arm, multicentre study of dabrafenib plus trametinib in patients with BRAFV600E-mutated BTC showed promising activity and a manageable safety profile (overall response rate [ORR]: 47%; n=43).26
Challenges in Delivering Precision Medicine in Cholangiocarcinoma
With molecular profiling, targetable alterations can be identified, but it is important to recognise the challenges associated with delivering precision medicine. Access to testing can often be difficult for patients and molecular profiling is not funded in every country; biopsies may not be of sufficient quality and quantity, and if the aim is to move treatment into the first-line setting then changes to recruitment criteria need to be addressed. Primary and secondary resistance need to be investigated in study trials to ensure that all patients gain benefit from starting one of these treatments.27 Approximately 40% of patients with BTC have targetable alterations, highlighting the importance of early patient testing, and a good supply of adequate tissue; many patients have a cytology-based diagnosis as molecular profiling is not available to them, and quality tissue samples are not always available resulting in failed samples.28
Best Practice in Genomic Testing in Cholangiocarcinoma
Nicola Normanno
Normanno led a discussion in strategic genomic testing in CCA, addressing the techniques used for genomic profiling and how the advancement of knowledge of common molecular alterations in BTC can be translated to clinical practice.14
Multiple genes and alterations require testing; point mutations, copy number alterations, and translocations all lead to gene fusions. Single biomarker tests using standard methods of detection, including quantitative PCR, fluorescence in situ hybridisation (FISH), and immunohistochemistry, are well known and widely used. Additionally, next-generation sequencing (NGS) can test for different types of genomic alteration in multiple genes.29 A major challenge is the difficulty in FGFR2 testing due to the limited number of options available. No immunohistochemistry technique has been developed and validated yet that can detect FGFR2 fusions, and reverse transcription-PCR is not feasible due to the high number of FGFR2 partners. Therefore, FISH and NGS are the only techniques available to test for FGFR2 fusions in iCCA.30
FISH Analysis
The FGFR2 break-apart probe approach is most commonly used to demonstrate the presence of FGFR fusion.31 Two fluorescently labelled DNA probes complementary to the 3’ and 5’ regions of FGFR2 genes are bound to two different dye colours (green and orange), providing a distinct coloured split signal on detection of FGFR2 rearrangement. However, some 50% of gene fusions in iCCA are intrachromosomal, occurring within the same chromosome as the FGFR fusion is located. Consequently, if the FGFR2 rearrangement is small, the FISH analysis may provide a false negative result.32
Next-Generation Sequencing Targeted Sequencing Panels
NGS permits a high number of nucleotides to be sequenced in a short time frame and is an affordable option.7 Targeted approaches using NGS allow isolation and sequencing of a subset of genes or regions of the genome and a number of different panels for targeted sequencing are commercially available. Panel size is variable, ranging from a few genes to hundreds of genes, and many companies can design panels to address specific research questions.7 Different technologies are available for library preparation, hybrid capture based, amplicon based, or Anchored Multiplex PCR, and panels are based on DNA and/or RNA sequencing for fusion detection.
Guideline Recommendations
Three levels of recommendations were proposed by the European Society for Medical Oncology (ESMO) Precision Medicine Working Group on the use of NGS in daily practice,7 based on perspectives obtained from public health, academic clinical research centres, and individual patients. In addition, the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) standardised how clinically relevant genomics data are reported and interpreted to ease implementation of precision medicine, and to provide an evidence-based classification system.33 Level 1 genomic alterations in advanced iCCA, according to ESCAT, are IDH1 mutations, FGFR2 fusions, MSI-H, and NTRK fusions, and based on this information. Recommendations by the ESMO on testing for genomic alterations in CCA state that “multigene tumour NGS could be recommended to assess Level 1 alterations.”7
Differences between DNA and RNA Sequencing
Both DNA and RNA-based NGS can be used to test patients with CCA and each of the libraries uses different nucleic acid panels for sequencing. DNA (hybrid capture-based),34 RNA (AMS),35 and DNA/RNA (hybrid capture-based36 and Amplicon based)37 panels are available. For DNA/RNA sequencing in the hybrid capture-based approach, the DNA panels are used for detection of mutations, insertions, and deletions, and copy number alteration, whereas the RNA panels are used to detect fusions. The Amplicon based approach using DNA/RNA panels uses DNA for mutations, insertions, and deletions, and copy number alteration, and RNA only for known fusions, and AMS allows better coverage regarding fusions as it is partner independent. DNA is more stable than RNA and there may be some instances where DNA is available for sequencing whereas the RNA has been degraded.
In determining the best time to test patients, it is assumed that driver genomic alterations relevant for tumour growth are mainly clonal, meaning that these alterations will be present in 1–2% of tumour cells.38 There is no evidence that chemotherapy might alter the frequency of clonal driver genomic alterations (IDH, BRAF, FGFR2) in CCA,39 and testing of patients with advanced CCA at diagnosis could maximise the possibility of patients receiving second-line treatment with targeted therapies.
Liquid Biopsy for Genomic Profiling of Cholangiocarcinoma
Liquid biopsy testing in cancer allows tumour-derived DNA, RNA, microRNA, and proteins (which can be either cell-free or contained in circulating tumour cells, extracellular vesicles, or platelets) to be investigated and analysed. Circulating cell-free DNA (cfDNA) testing has many advantages compared with tissue testing, but is also met with challenges. Testing is highly compliant and minimally invasive, and accounts for tumour heterogeneity at primary and metastatic sites. It is easily monitored with a reduced turnaround time compared with tissue testing. However, only a few nanograms are isolated per mL of plasma, so the absolute levels are low and are often correlated with tumour burden. Levels are also usually higher in patients with advanced disease. Tumour-derived cfDNA contains both circulating tumour DNA and normal DNA originating from blood cells, the gastrointestinal (GI) tract, and skin; circulating tumour DNA comprises <0.1–50.0% of cfDNA. With a short half-life of 90 days, all of these challenges make liquid biopsy very difficult to test.40 In cfDNA analysis, analytical sensitivity varies according to different approaches and limits of detection show sensitivity up to 0.001% with emulsion PCR-based technologies, for example.40
Emerging Therapies for Previously Treated Advanced Cholangiocarcinoma
Arndt Vogel
Preclinical studies have shown that independent activation of the FGF receptors (FGFR1–4) by FGF ligands (FGF 1–10 and FGF 16–23) can lead to tumour development.41-43
Phase II Studies in Pre-Treated Patients
Pemigatinib: efficacy data
One of the most advanced drugs to date is pemigatinib and the FIGHT-202 Phase II, single-arm, open-label, multicentre study evaluated the efficacy and safety of pemigatinib in patients with locally advanced/metastatic/surgically unresectable CCA.44 Enrolled participants were assigned to one of three groups: Cohort A (n=107; FGFR2 fusions/rearrangements), Cohort B (n=20; other FGF/FGFR genetic alterations), or Cohort C (n=18; no FGF/FGFR genetic alterations; a single patient had an undetermined FGF/FGFR genetic alteration). Patients were treated with oral pemigatinib 13.5 mg once daily (2 weeks on, 1 week off) until disease progression or toxicity, and median follow-up was 17.8 months (interquartile range: 11.6–21.3). The primary endpoint was a confirmed ORR in Cohort A by an independent central review.45
In Cohort A (n=107), 56 different fusion partner genes were detected among 92 fusions and 15 rearrangements, and of those partner genes, 42 were unique to single patients. The most frequently observed fusion was BICC1 (n=31, 29%) and it is the variability of fusion partners that makes FGFR2 fusion detection more challenging. Vogel stressed the importance of ensuring that the correct molecular pathology test is being applied to capture all patients with FGFR2 fusions. Demographically, Cohort A had more female patients and were of a younger age (61%; 77% <65 years) than Cohorts B (55%; 50% <65 years), and C (44%; 39% <65 years).
Updated results46 presented at the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting showed the ORR for Cohort A (n=108, one additional patient from Japan was enrolled in Cohort A after the primary cutoff date as they were already in screening) was now 37% in these pretreated patients with FGFR2 fusions (Figure 2)
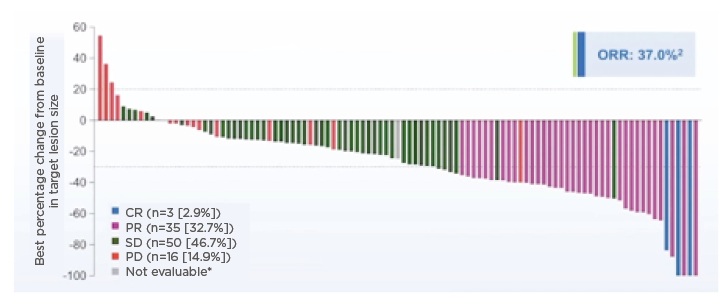
Figure 2: Change from baseline in target lesion size for Cohort A (overall response rate: 37%).
*Patient showed a decrease in target lesion size but not evaluable for response using RECIST.
CR: complete response; FGFR: fibroblast growth factor receptor; ORR: objective response rate; PD: progressive disease; PR: partial response; SD: stable disease.
Adapted from Abou-Alfa et al., 202045 and Abou-Alfa et al., 2021.46
Efficacy was independent of the lines of prior treatment and independent of FGFR2 rearrangement partners. A total of four patients had complete response, 36 had PR, the median duration of response was 8.1 months, median PFS was 7 months, and median OS was 17.5 months. A remarkable disease control rate of >80% was achieved and Figure 2 demonstrates how only a few patients had progressive disease, which is promising in the second- and third-line setting. It is important to remain cautious with OS data until evaluation has taken place over a longer time. Nevertheless, the updated OS analysis showed that for patients with FGFR2 fusions or rearrangements, the median OS for patients who responded to pemigatinib with either a complete response or PR was a remarkable 30.1 months compared with 13.7 months for those who did not respond to pemigatinib, although 13.7 months is still a meaningful survival result.46
Pemigatinib: safety data
Treatment-emergent adverse events occurred in ≥25% of the overall patient population,46 and the safety profile in the updated analysis was consistent with the primary analysis with no new safety signals.45 Most patients developed Grade 1 or Grade 2 toxicity for a range of adverse events and there was no Grade 3 hyperphosphataemia. GI and skin toxicities were observed in patients although these were manageable and led to discontinuation of the drug in only a few patients (13/146, 9%).46
Additional Phase II and III studies in FGFR2 fusion positive patients (previously treated)
Efficacy results for infigratinib,47 derazantinib,48-50 and futibatinib,51 show sufficiently consistent data for ORR, disease control rate, and PFS to indicate that a class effect is present. Ongoing Phase III studies24,25,52 may reveal differences in efficacy and safety data with time, but currently, the data support the continuation of targeted therapies in FGFR2 fusion-positive patients.
Secondary resistance in cholangiocarcinoma
Secondary resistance develops when secondary FGFR2 mutations occur, and as patients develop FGFR2 kinase domain mutations they become drug-resistant with tumour progression.53 A study of futibatinib (TAS-120), a selective and irreversible small-molecule inhibitor of FGFR1-4, demonstrated efficacy in patients with FGFR2 fusion-positive iCCA who had developed resistance to ATP-competitive FGFR inhibitors.53 Futibatinib showed activity in vitro against all mutations with the exception of the gatekeeper residue V565F, and in silico structural modelling indicated that steric hindrance of the dimethoxy phenyl group may prevent access to the ATP-binding pocket.53 For the patient, this usually translates to an inability to respond to any further FGFR inhibition.
Neratinib and trastuzumab/pertuzumab in biliary tract cancers: efficacy data
HER2 is a genetic target which is increasingly recognised within GI oncology, and the first data on HER2 alterations in BTC are now available. Interestingly, HER2 alterations are not specific to iCCA and patients may present with eCCA or gallbladder cancer. Additionally, BTC patients are also observed to have activating HER2 mutation. A Phase II study of neratinib (N=25) in patients with BTC,54 a pan-HER irreversible tyrosine kinase inhibitor, showed an ORR of 16% (4/25) with some patients showing a deep response. In the MyPathway Phase II trial of trastuzumab/pertuzumab (N=11) in patients with BTC, a subset analysis of preliminary results showed ORR (amplified/overexpressed) of 37.5% (3/8) and ORR (mutated) of 33.3% (1/3).55
Summary
Genomic alterations with potential therapeutic implications have been identified in around half of all patients with CCA, leading to a focus on precision medicine in emerging therapies for previously treated, advanced CCA. Biomarkers are key for the development of precision medicine strategies in CCA, and there are already known targetable alterations of relevance.14 To bring precision medicine to the clinic, however, there needs to be early testing and adequate tissue or biopsy samples to enable identification of other rare alterations that could result in matching individuals with a specific treatment option.26 NGS is recommended by the ESMO guidelines as a technique for comprehensive genomic profiling,7 and patients should be tested at the time of diagnosis even if their disease is locally advanced. DNA and RNA sequencing methods have many pros and cons associated with both, although RNA sequencing allows for better coverage of fusions. Ultimately, more data are needed to determine whether FGFR2 fusions are of prognostic value or if they are purely a predictive marker of FGFR inhibitors and secondary resistance will be the next challenge. In emerging therapies, clinical trials are showing promising results for targeted therapies in CCA, with several Phase III studies underway.24,25,52,56 However, a minority of patients do not respond well to specific drugs within a class, even though these are targeted therapies within a genetically defined group of patients. Thus, there is a need for more biomarkers to enable selection of the right patients for the right drug.








