Interviewees: Maximilian J. Hochmair,1 Umberto Malapelle2
1. Department of Respiratory and Critical Care Medicine, Karl Landsteiner Institute of Lung Research and Pulmonary Oncology, Vienna, Austria
2. Department of Public Health, University of Naples Federico II, Italy
Disclosure: Hochmair has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda Pharmaceutical Company, and Roche; and has had consulting or advisory roles with Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Takeda Pharmaceutical Company, and Roche. Malapelle has received speaker and consultant fees from Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Amgen, Thermo Fisher Scientific, Eli Lilly and Company, Diaceutics, Diatech Pharmacogenetics, GlaxoSmithKline, Merck Sharp & Dohme, and AstraZeneca.
Acknowledgements: Medical writing was provided by Bronwyn Boyes, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the two named interviewees.
Support: The publication of this interview feature was supported and reviewed by Novartis.
Citation: EMJ Oncol. 2022;10(Suppl 5):2-9.
Interview Summary
Non-small cell lung cancer (NSCLC) is a heterogeneous disease with many genomic mutations.1 Furthermore, comprehensive genomic testing is now the standard of care to identify common or uncommon actionable genomic alterations that impact therapeutic decision making.2,3 Guidelines recommend testing for molecular and immune biomarkers in patients with advanced or metastatic NSCLC to assess eligibility for targeted therapy or immunotherapy as these have demonstrated greater efficacy when compared to chemotherapy.4-7 The mesenchymal–epithelial transition (MET) is a tyrosine kinase receptor that is mostly expressed in epithelial cells, whose natural ligand is the hepatocyte growth factor. MET signalling involves cell proliferation, migration, invasion, and survival.8 Genomic alterations in MET include MET exon 14 skipping mutations (METex14) or activating mutations, MET gene amplification, and MET protein overexpression.9 However, the presence of METex14 mutations is currently the best-defined predictive biomarker for the use of MET tyrosine kinase inhibitors (TKI). The METex14 mutation has a prevalence of approximately 3%, representing approximately 4,000–5,000 patients with metastatic NSCLC per year in the USA. The prevalence of METex14 is similar to that of other genomic mutations, such as ALK fusions (approximately 3–4%), and BRAF mutations (approximately 3–4%).10-17 Patients with METex14 in metastatic NSCLC face a poor prognosis.18-20 Many of these patients may have bone, liver, and brain metastases, which are associated with poor outcomes.12-14 Advances in the understanding of these mutations have led to new approaches to the diagnosis and the development of MET kinase inhibitor therapies, enabling personalised treatment and improved outcomes for patients with NSCLC. In this article, two leading experts discuss the latest evidence on MET testing and inhibitors in NSCLC. These valuable insights were obtained from an interview conducted by the EMJ in December 2021.THE EVOLVING LANDSCAPE IN TESTING FOR MET EXON 14 SKIPPING MUTATION IN NON-SMALL CELL LUNG CANCER
Umberto Malapelle
Malapelle started by explaining that a panel of so-called must-test genes need to be tested as a minimum in patients with advanced stage NSCLC before the selection and initiation of targeted therapies.1 This is according to established guidelines from the College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), Association for Molecular Pathology (AMP),2,3 American Society of Clinical Oncology (ASCO), Ontario Health (OH), Cancer Care Ontario (CCO),4 European Society for Medical Oncology (ESMO),5,6 and National Comprehensive Cancer Network (NCCN).7 The panel includes EGFR and BRAF mutations, ALK, ROS1, RET, and NTRK1/2/3 gene rearrangements, and METex14 skipping. In addition, programmed death-ligand 1 (PD-L1) expression evaluation is required for the administration of immunotherapy regimens.1-7 Beyond these approved biomarkers, other gene alterations (e.g., KRAS exon 2 p.G12C, ERBB2, and MET amplifications) are currently under investigation.4,5,8 In addition, in order to avoid inadvertently missing any patients, the guidelines strongly recommend performing molecular testing not only on non-smoker, younger, adenocarcinoma histology patients with NSCLC, but in all advanced stage patients with NSCLC as a part of a broad molecular profiling.6-8
However, tissue samples remain an issue in patients with NSCLC.10,11 In fact, in a non-negligible percentage of patients, the only available tissue material for morph-molecular evaluation is represented by small tissue samples (e.g., biopsies or cytological specimens). In this setting, the adoption of next generation sequencing (NGS) methodologies may be an optimal solution to maximise the scant tissue material for molecular testing.12 NGS ensures the analysis of different biomarkers (either DNA- or RNA-based) for different patients, even starting from a very limited nucleic acids input.12,13 Liquid biopsy is another option to overcome tissue availability. Currently in clinical practice, this term refers to the analysis of circulating tumour DNA extracted from plasma.10 The adoption of circulating tumour DNA has been approved into two clinical settings: in patients with advanced NSCLC naïve to any treatment (basal setting) without tissue availability, or with an inadequate molecular result for the identification of EGFR gene alterations in order to administer first or second generation epidermal growth factor receptor (EGFR) TKIs; at resistance to a first or second generation EGFR TKI regimen for the identification of EGFR exon 20 p.T790M resistance point mutation; and for the administration of the third generation EGFR TKI, osimertinib.10,14 As reported by the CAP, IASLC, and AMP guideline, the adoption of a ‘blood first’ approach is strongly encouraged for molecular testing evaluation in order to save tissue specimens to test negative samples, and for the analysis of tissue-based biomarkers (such as PD-L1).2
Malapelle outlined that it is fundamental to identify the specific molecular alteration of each patient before initiating any treatment. This is crucial as targeted drugs can only exploit their action in the presence of a specific target.15 Without a specific target, not only are targeted drugs pointless but they can also have a detrimental effect on patients.16 This has been widely demonstrated in patients with a high PD-L1 expression harbouring contemporary driver mutations who did not benefit from immunotherapy.17
To provide access more broadly to new testing technologies, Malapelle believes that smaller centres should be integrated in networks managed by academic institutions. In this way, peripheral and small centres that require molecular testing directly send tissue material or liquid biopsy specimens to the referral centres for a centralised molecular analysis. In this scenario, it is fundamental that the referral centre provides effective communication and education, to ensure an adequate sharing of knowledge among the different institutions within the network. In addition, the adoption of a knowledge database, such as Atlas, OncoKB, or similar may be useful.18-20
Malapelle then focused on METex14 skipping alterations, which can be detected in 3–4% of patients with NSCLC.21-23 Thus, not a negligible percentage of patients with advanced stage NSCLC may benefit from a targeted TKI treatment. The inclusion of METex14 skipping alterations analysis in the diagnostic algorithm of patients with advanced stage NSCLC is crucial to prevent undertreatment.
MET represents a different approach from a testing point of view, with three different types of alterations: point mutation that can cause skipping in the exon 14; MET expression that requires evaluation by immunohistochemistry; and copy number variation of the MET gene requiring fluorescence in situ hybridisation or NGS assay.24
METex14 skipping alterations may be detected through DNA- or RNA-sequencing (Table 1). DNA-sequencing has the advantage of adopting DNA as the nucleic acid starting material, which is more stable compared with RNA and is easier to manage for molecular purposes. However, a disadvantage of DNA-sequencing is the presence of large introns that are typically inadequately sequenced and difficult to analyse. RNA-sequencing is performed on messenger RNA. Despite the disadvantage represented by the reliance on RNA quality, which can be poor if obtained from formalin-fixed and paraffin-embedded samples, it does not suffer from the presence of large intronic regions.25-29
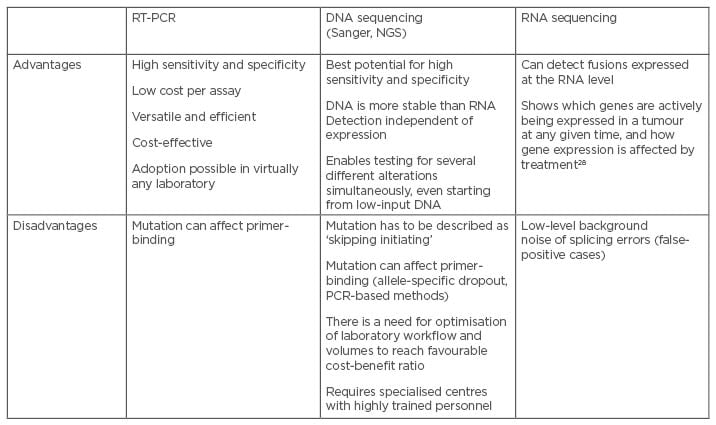
Table 1: Advantages and challenges associated with METex14 testing methods.25-28
NGS: next-generation sequencing; RT-PCR: real-time polymerase chain reaction.
METex14 skipping testing is currently part of the standard of care for patients with NSCLC (Figure 1).30,31 More recently, capmatinib was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with advanced stage NSCLC harbouring METex14 skipping mutations. This was based on the results of the GEOMETRY mono-1 trial (NCT02414139),32 a multicentre, non-randomised, open-label, multicohort study enrolling patients with metastatic NSCLC with confirmed METex14 skipping (Table 2).33 In addition, tepotinib has also obtained FDA approval for the treatment of patients with advanced stage NSCLC harbouring METex14 skipping, following the results of VISION trial (NCT02864992).34 This was a multicentre, non-randomised, open label, multicohort study enrolling patients with advanced or metastatic NSCLC with METex14 skipping alterations (Table 2).35 Thus, it is pivotal to consider METex14 skipping as part of the must-test genes to optimise selection and management of patients with advanced stage NSCLC.
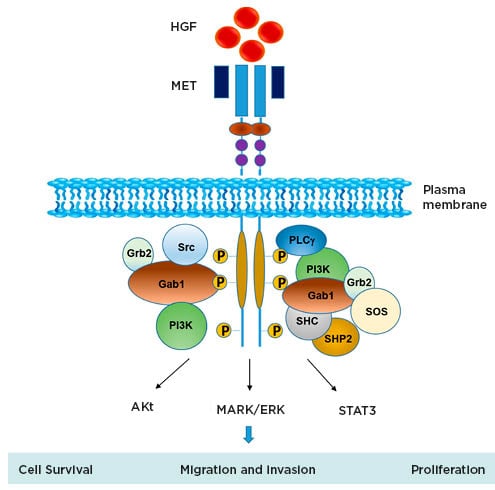
Figure 1: Schematic representation of MET signalling.
Figure from Owusu BY et al.30 Akt: protein kinase B; HGF: hepatocyte growth factor; MAPK/ERK: mitogen-activated protein kinases/extracellular signal-regulated kinases; MET: mesenchymal–epithelial transition; STAT3: signal transducer and activator of transcription 3.
Malapelle concluded by explaining that close collaboration among oncologists, pathologists, and molecular biologists is crucial to achieve improved outcomes in terms of diagnosis and treatment choice in patients with NSCLC. He believes it is fundamental to discuss all cases, in particular complex cases, within molecular tumour boards. Furthermore, a timely molecular diagnosis is another crucial point in the management of patients with advanced stage NSCLC. Following the international guideline recommendations, the time between an oncologist requesting molecular testing and the final integrated morph-molecular report becoming available should not exceed 10 working days.36
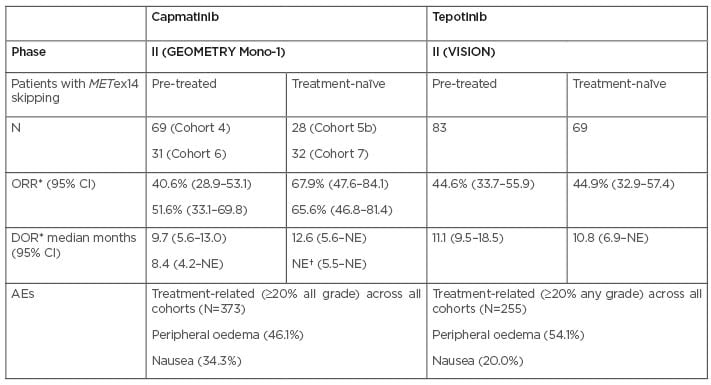
Table 2: Summary of findings from clinical trials with two key mesenchymal–epithelial transition inhibitors used as monotherapy in non-small cell lung cancer.32-36,48,51-53
*BIRC or IRC.
†Not yet mature.
AE: adverse event; BIRC: blinded independent review committee; CI: confidence interval; DOR: duration of response; GCN: gene copy number; IRC: independent review committee; NE: not estimated; ORR: overall response rate.
THE EVOLVING TREATMENT LANDSCAPE IN MET EXON NON-SMALL CELL LUNG CANCER
Maximilian Hochmair
Hochmair outlined that all his patients with NSCLC are molecular tested, irrespective of the subtype. Initially in 2018, it was believed that biomarker targets would not be found in all patients, so only non-squamous and non-smoking patients with NSCLC were tested. However, this changed in 2020 to test all patients with NSCLC, because druggable targets have been identified in patients with squamous NSCLC. It is now the recommendation of the Austrian Working Group on Pulmonary Pathology and Oncology (AWGPPO) to test all patients, independent of staging, to save time.37 Biomarker analysis demonstrates that each patient with lung cancer is unique, and, as such, a full biomarker analysis even in the early stages of NSCLC has a clinical impact (for example, approved use of osimertinib for EGFR mutated NSCLC,38 as well as investigational targeted therapy for RET39 and ALK40 NSCLC in the adjuvant setting). Furthermore, in patients with early recurrence post-surgery or chemoradiotherapy, it is important to understand if there are any druggable biomarker targets.
Hochmair added that simultaneous separate testing of different targets is no longer conducted, moving to NGS testing for MET and other targets like RET, HER2, EGFR, ALK, ROS1, and KRAS. The obvious benefit of testing for all targets upfront and not sequentially is to obtain faster results, and to save tissue samples for the pathologist.
Malapelle concurred on the importance of testing an entire panel of biomarkers, which, if not adhered to, can cause problems with the testing strategy. For example, if METex14 skipping alterations are analysed using a real-time PCR approach, without consideration of other relevant biomarkers such as EGFR, BRAF, KRAS, etc., this can cause problems when needing to produce an entire identity of clinically relevant biomarkers for these patients.
Biological Characteristics
Hochmair went on to explain that there are no typical characteristics for patients with METex14 skipping NSCLC. Therefore, it is imperative not to exclude patients based on clinical characteristics for biomarker testing. Patients enrolled in the capmatinib trial at Hochmair’s institution were typically older patients (>75 years), with slightly more females with comorbidities. However, typically older patients have comorbidities, so comorbidities may not be a predictor for METex14 skipping mutation. Conversely, Hochmair has also treated younger patients without comorbidities, who have never smoked, with METex14 skipping NSCLC, highlighting the importance of testing all patients with NSCLC.
Malapelle commented that historically younger, female patients were typically seen with the EGFR-mutation. However, it is really important to adhere to guideline recommendations to test all patients with NSCLC,1-7 and not to select patients for testing based on clinical characteristics, as this can result in a reduction in the patient population that could be elected for this potentially efficacious targeted treatment.
Another important point noted by Hochmair is that older patients with comorbidities are not always able to tolerate toxic chemotherapy or combination chemotherapy with an immune-oncology (IO) therapy. Therefore, there is a significant medical need for these patients to receive a targeted therapy that may be better tolerated, with fewer toxicities, and with good efficacy. In Hochmair’s experience, a MET inhibitor such as capmatinib is preferred as a first-line option for these patients. Furthermore, based on his experience and also retrospective analysis, immunotherapy is not as efficacious in these patients, even when PD-L1 is high.41-44
Importance of MET as a Target for Non-Small Cell Lung Cancer
Malapelle highlighted that the METex14 skipping mutation is a significant oncogenic driver in patients with NSCLC, with five times the prevalence of RET (1.0%), NTRK (0.7%), and ROS1 (1.0%).45 Therefore, MET represents a significant target in this setting, with efficacious treatment options. Hochmair agreed and noted that the prevalence of the METex14 skipping mutation from the Austrian patient registry is approximately 3% in patients with NSCLC. Malapelle then explained that the prevalence of the alteration is related to the type of assay used. When using a real-time PCR approach, the real prevalence is less than the theoretical prevalence, as the test is unable to cover all the clinically relevant alterations.
Hochmair advocated for the use of MET inhibitors in patients with METex14 skipping NSCLC. He believes that the efficacy and tolerability of chemotherapy and IO for METex14 NSCLC is inferior. Therefore, MET inhibitors can aid improved outcomes in these patients. The two main options include capmatinib and tepotinib.46-50
The VISION trial (NCT02864992)34 was a multicentre, non-randomised, open label, multicohort study, enrolling 152 patients with advanced or metastatic NSCLC with METex14 skipping alterations (Table 2). Patients received tepotinib 450 mg orally once daily until disease progression or unacceptable toxicity were noted. The main primary and secondary efficacy outcome measures were overall response rate, determined by a blinded independent review committee using RECIST 1.1 and response duration. The most common adverse events (AE) occurring in the safety population (N=255) were oedema, fatigue, nausea, diarrhoea, musculoskeletal pain, and dyspnoea (≥20% of patients).34,35
The GEOMETRY mono-1 trial (NCT02414139),32 was a multicentre, non-randomised, open-label, multicohort study, enrolling 160 patients with metastatic NSCLC with confirmed METex14 skipping (Table 2).49 Patients received capmatinib 400 mg orally twice daily until disease progression or unacceptable toxicity. The primary efficacy outcome measure was overall response rate, determined by a blinded independent review committee using RECIST 1.1, and response duration as a secondary outcome. The most common AEs (≥20% of patients) in the safety population (N=373) were peripheral oedema, nausea, fatigue, vomiting, dyspnoea, and decreased appetite.30,33,49
Peripheral oedema and gastrointestinal toxicity are recognised as class effects associated with MET inhibitors. These may be managed with a dose interruption, dose reduction, or permanent discontinuation if unresolved.54,55 In Hochmair’s experience, the use of diuretics is not very helpful for oedema; however, compression stockings may be useful. Patient education is also important when managing AEs.
In conclusion, both experts believe that the efficacy of targeted therapy relies on oncogene addiction. When compared with chemotherapy and IO, MET TKIs have demonstrated robust clinical efficacy with manageable safety profiles, and have the ability to improve outcomes in patients with METex14 NSCLC.

![EMJ Oncology 10 [Supplement 5] 2022 Feature Image](https://www.emjreviews.com/wp-content/uploads/2022/03/EMJ-Oncology-10-Supplement-5-2022-Feature-Image-940x563.jpg)






