Interviewees: Axel Hauschild,1 Salvador Martín Algarra2
1. Department of Dermatology, University of Kiel (UKSH), Kiel, Germany
2. Medical Oncology, Clinica Universidad de Navarra, Pamplona, Spain
Disclosure: Prof Dr Hauschild has received clinical trial support from Amgen, Bristol Myers Squibb, Merck Serono, MSD, Novartis, Philogen, Pierre Fabre, Provectus, Regeneron, and Roche; speaker’s honoraria from Amgen, Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, Provectus, and Roche; and consultancy fees from Amgen, Bristol Myers Squibb, Merck Serono, MSD, Novartis, OncoSec, Philogen, Pierre Fabre, Provectus, Regeneron, and Roche. Dr Algarra has received clinical trial support from Amgen, Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, and Roche; speaker’s honoraria from Amgen, Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, and Roche; and consultancy fees from Amgen, Bristol Myers Squibb, Merck Serono, MSD, Novartis, Pierre Fabre, Regeneron, Roche, and Sanofi.
Acknowledgements: Medical writing assistance was provided by Dr Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Citation: EMJ. 2021;6[1]:15-22.
Interview Summary
Nonmelanoma skin cancers (NMSC) are a diverse group of cutaneous malignancies and are the most common forms of human neoplasia worldwide.1 The incidence of these diseases has increased during the last three decades2,3 and there are up to 3 million new cases of NMSC every year.4 There are geographical variations in incidence based on different ultraviolet (UV) exposure rates, with the highest incidence of NMSC in Australia.3 The incidence of NMSC is increasing and yet these cancers are considered to be neither clinically, nor from a research perspective, as relevant as other tumours. The majority of NMSC are basal cell carcinomas (BCC) and cutaneous squamous cell carcinomas (CSCC). Advanced (locally advanced and metastatic) BCC and CSCC are associated with poor outcomes and are underserved in terms of treatment. Medical therapy improvements in advanced NMSC have not occurred as rapidly as those seen in melanoma, and there is a clear unmet need in the systemic treatment of patients with NMSC.
For this article, the EMJ conducted interviews in July 2020 with two key opinion leaders, Prof Dr Axel Hauschild from Germany and Dr Salvador Martín Algarra from Spain, both of whom have a wealth of experience and expertise in managing NMSC, to gain their perspectives on a range of topics in this area. The experts gave valuable insights into several pertinent issues in NMSC treatment and discussed significant recent developments in the field.
The article discusses the incidence of and current treatment landscape for NMSC and highlights the unmet need in the treatment of these diseases. New biological therapies with a different mechanism of action and their inclusion in a treatment algorithm for previously difficult-to-treat patients are considered. Screening and prevention of these diseases are also explored.
NONMELANOMA SKIN CANCERS: INCIDENCE AND PROFILE
Increasing Incidence of Nonmelanoma Skin Cancers
Prof Hauschild explained that the major reason for the increasing incidence of NMSC, particularly CSCC, is changing UV exposure patterns, and that individuals with high sun exposure 30–40 years ago are now presenting with actinic keratosis, precancerous lesions, and invasive CSCC. He emphasised that: “Patients are not developing only one tumour, but multiple primary tumours.” These tumours form anywhere on the body but 80% occur in sun-exposed areas, such as the face and ear edges and, in males with androgenic alopecia, on the scalp. Prof Hauschild considered a minor reason for increasing NMSC incidence is the growing numbers of immunosuppressed patients, particularly organ transplant recipients, who have multiple NMSC, particularly CSCC, because immunosuppression is allowing the tumours to grow rapidly; these patients may present with more advanced disease.5
According to Dr Algarra, “NMSC is a very serious disease that is growing in incidence due to several known factors, including greater leisure exposure to the sun,6,7 but there are several other factors that also need to be considered. Human papillomavirus8 and other viruses could also play a role in the incidence and development of these diseases.” He also considered that age is another very serious factor because there is a growing elderly population and the incidence of these tumours increases with age.9
Dr Algarra highlighted the increasing incidence of NMSC with higher ambient air pollution. A study of routine healthcare data from around 1.9 million people in Saxony, Germany, showed an increase in particulate matter with aerodynamic diameter of <10 μm (PM10) was associated with a 52% increase in relative risk of NMSC.10
Statin use has also been associated with higher incidence of NMSC,11 which Dr Algarra suggested is “something quite provocative,” particularly as the use of statins is increasing. Also, Dr Algarra mentioned that additional factors, such as socioeconomic or smoking status, genetic predisposition, pesticides, or other commonalities of the modern lifestyle, may be relevant to the growing incidence of these tumours.
The Major Difference Between Nonmelanoma Skin Cancers Is the Tendency to Metastasise
Prof Hauschild explained that BCC almost never metastasises (he has seen only two cases of metastatic BCC in his 30-year career), is locally aggressive, and could, in principle, invade bones (this is very rarely observed in CSCC). The incidence of locally advanced BCC has been reported as 0.8% in a large USA study,12 whereas a Danish study showed a 14-year cumulative incidence proportion of metastatic BCC of 0.0039% among individuals with a history of previous BCC and 0.0001% in the general population.13
The major difference between BCC and CSCC is the tendency for the latter to metastasise, with metastatic CSCC observed as often as locally advanced disease. The most important risk factor for metastatic CSCC is maximum (vertical) tumour thickness.14 Tumour thickness of >6 mm equates to a 30% chance of metastases, <2 mm thickness equates to 0%, and 2–6 mm thickness has a rating between 0 and 30%. Immunosuppression, localisation on the lip or ear, differentiation grade, and perineural invasion are also risk factors.
Why Do Nonmelanoma Skin Cancers Have a Lower Profile than Melanoma Skin Cancers?
When asked why NMSC are not as often discussed or as high profile as melanoma skin cancers, Prof Hauschild explained: “The incidence of NMSC is very high, with BCC the most common tumour in humans followed by, most likely, CSCC. There is less research in NMSC compared with melanoma because cases of locally advanced and metastatic NMSC are very rare, representing about 2% of CSCC patients. In the vast majority of cases, you are excising the lesions, so the surgery is doing the job.”
Continuing this theme, Dr Algarra explained that the incidence of melanoma is lower than that of NMSC, but melanoma is a more aggressive disease. Dr Algarra considered: “There are biological reasons that make these tumours [NMSC] less dramatic and, therefore, not as relevant to general public awareness. Furthermore, local measures cure NMSC in the large majority of patients, NMSC is more common in ageing populations (melanoma is more common in younger populations), and those rare cases that metastasise usually do not behave in such an aggressive way as melanoma. All these facts make cutaneous melanoma more relevant than NMSC for the news, the media, the clinicians, and the researchers.” He continued: “Nevertheless, it is very important to consider that there is a subset of NMSC patients who cannot be treated with local measures and progress to mutilating or metastatic disease, with very limited or no treatment options. These particular patients deserve as much attention and medical commitment as those with advanced melanoma or Merkel cell carcinoma.”
The Unmet Need in Treatment of Nonmelanoma Skin Cancers
Dr Algarra described a “darker phase” in NMSC: “When NMSC relapses after one or several surgeries, it is well proven that radiation therapy could help to control the disease, but there is a considerable number of patients who relapse, progress after radiation therapy, are not surgically treatable, and may be wrongly considered not suitable for any treatment.” Dr Algarra emphasised: “Advanced NMSC patients, particularly those with BCC or CSCC, are sometimes neglected. We have experienced that in other challenging diseases. NMSC patients deserve a serious commitment from a basic, translational, and clinical research point of view.” He explained that most professionals in the field accept that sometimes there is “nothing you can do after local treatment,” but these usually elderly patients, who may not be able to care for themselves or have other medical and psychological difficulties, “deserve an evidence-based medical approach that considers the pros and cons of active treatments.”
TREATMENT LANDSCAPE
Although Dr Algarra considered NMSC to be a group of diseases that may not be as relevant for the medical oncology community as breast cancer, prostate cancer, or melanoma, he reiterated that it is a very serious matter that needs to be approached with enthusiasm and professionalism. He stated: “Recent advances in biology, translational research, and clinical research have shown clearly that these patients can be treated and may benefit from the newest approved agents. We need to develop not only algorithms but also attitudes to confront the disease in a very serious way.”
Locally Advanced Disease: A Relatively New Concept
Prof Hauschild explained that in major guidelines worldwide, such as the National Comprehensive Cancer Network (NCCN)15 and the European Association of Dermato-Oncology (EADO)14,16 guidelines, the first treatment option is the excision of the primary tumour, and that in CSCC and BCC there is a relatively new term, ‘locally advanced’, which is not well defined. “The interdisciplinary tumour board says we can excise or irradiate the tumour, but it will be very difficult,” he explained, “so if there is an alternative, such as systemic treatment, they would prefer the systemic treatment, and this is how the hedgehog pathway inhibitors (HPI) sonidegib and vismodegib have been developed for advanced BCC, and why cemiplimab and other programmed cell death receptor 1 (PD-1) antibodies have been introduced to the field for CSCC.”
Introduction of a Different Mechanism of Action: Programmed Cell Death Receptor 1 Inhibitors in Nonmelanoma Skin Cancers
PD-1 inhibitors are new biological therapies with a different mechanism of action: they bind to PD-1 and block its interaction with programmed death ligands 1 (PD-L1) and 2 (PD-L2), representing a new treatment pathway for NMSC.17-19
Clinical responses to the PD-1 inhibitor cemiplimab (Libtayo® [Sanofi, Paris, France], the first medicine approved by the U.S. Food and Drug Administration [FDA] and European Commission for CSCC that has spread or cannot be cured by surgery or radiation20) in patients with advanced CSCC who were not candidates for curative surgery or radiation therapy have been shown in Phase I and II studies,21-23 with longer-term results presented at the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting showing durable responses that deepened over time.24,25 Across all groups combined, with 15.7 months’ median duration of follow-up, complete response (CR) rates were 16.1% (n=31) and in the metastatic group with the longest follow-up (Group 1 in Table 1), the CR rate was 20.3% (n=12), increased from 6.8% (n=4) in the 2017 primary analysis.21,25,26
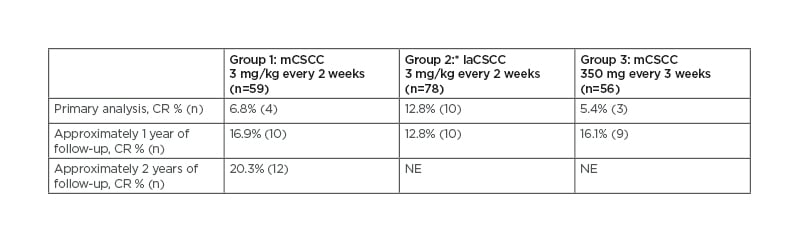
Table 1: Duration of follow-up and tumour response to cemiplimab.
*Among 23 patients with laCSCC who were included in the prespecified Group 2 interim analysis, there were no CR.
CR: complete response; laCSCC: locally advanced cutaneous squamous cell carcinoma; mCSCC: metastatic cutaneous squamous cell carcinoma; NE: not evaluable.
There has also been a recent focus on cemiplimab in patients with advanced BCC who had progressed on, or were intolerant of, prior HPI therapy. In a pivotal, single-arm, open-label study,28 objective responses were seen in 29% of patients with locally advanced BCC and in 21% of patients with metastatic BCC, with approximately 85% of patients who responded to cemiplimab maintaining their response for at least 1 year.27
Where do Hedgehog Pathway Inhibitors and Programmed Cell Death Receptor 1 Inhibitors Fit into the Treatment Landscape for Nonmelanoma Skin Cancers?
Prof Hauschild noted that the guidelines specify excision, with irradiation a further option if excision is not feasible; however, nowadays very few tumours are irradiated. “For locally advanced and, in particular, metastatic BCC, in the past there were HPI in first-line,16 and there was no second-line treatment. Following the press release27 from Regeneron [Tarrytown, New York, USA] and Sanofi on cemiplimab20 in BCC, cemiplimab will potentially become a second-line treatment of choice for BCC. For CSCC, cemiplimab has replaced chemotherapy and any sort of epidermal growth factor receptor (EGFR) inhibitor treatment such as cetuximab,” he outlined.
The introduction of PD-1 inhibitors has created a change in clinical practice and guidelines. Prof Hauschild explained: “Cemiplimab is now the treatment of choice for patients with locally advanced or metastatic CSCC, and this is good because we needed something that is more effective than chemotherapy and EGFR inhibitors, with few and mainly short-lasting responses.” Furthermore, the introduction of PD-1 inhibitors in BCC gives physicians another treatment choice for patients in whom the options after failure on HPI are very limited. Prof Hauschild declared: “In general, we should give the best available agents in first line and not in second line; we should not wait for the progression of the tumour.” Furthermore, Prof Hauschild considered that “the data and the patient selection in clinical trials for CSCC reflect the real world.”
Dr Algarra acknowledged that several recent advances in the field have totally changed the treatment paradigm for NMSC and attempts to rescue with other local treatments, such as electrochemotherapy,29-31 are no longer the only tools to overcome these diseases. “We have to be prepared to use these new treatments, which have proved to be active even in situations that were qualified in the past as unsurmountable.”
Dr Algarra specified that a clear algorithm is needed to show how to use HPI and PD-1 inhibitors: “We must be aware that the indications for these treatments are going to grow because they are approved and recognised as active in metastatic disease.” He clarified: “They must also be considered in locally advanced disease to avoid mutilation, and even in less advanced disease if the tumour location compromises the physical and psychological health of the patient.” Dr Algarra envisions there will be room for adjuvant treatment:32 “We have to move from the current approval in advanced disease to the not so uncommon high risk of relapse scenario. In this sense we are eagerly awaiting the results of the clinical trials.”
When asked about the integration of biological treatments and immunotherapy into the treatment algorithm for NMSC, Dr Algarra responded that from a real-life point of view, it may be a challenging task because of the need to consider resectability, which depends on the size and location of the tumour, number of prior relapses, and local treatment used; patient-related issues, such as comorbidities, dependence, and socio-labour needs; and regulatory issues, such as local agencies’ approval, costs, hospital regulations, and pharmacy and multidisciplinary team consensus.
Treatment Options for Patients with Basal Cell Carcinomas Who Progress on Hedgehog Pathway Inhibitors
According to Prof Hauschild, “the only option at the moment for BCC patients is to be treated with PD-1 inhibitors in clinical trials or as an off-label treatment. Now that cemiplimab has shown a 29% response rate in second line and 85% of the responses are stable for at least 1 year,27 it is very clear that a PD-1 inhibitor like cemiplimab is likely to be approved and we ought to ask our payers/insurance companies to get such a treatment in this setting. Cemiplimab is doing a good job here and I would love to see studies in first line but they are not currently available.” He listed other treatment options as chemotherapy, irradiation, or best supportive care.
In terms of timing for PD-1 inhibitor treatment in patients with BCC, Prof Hauschild clarified: “I would give the HPI a chance for at least 8–12 weeks, by which time you will know if the patient is responding. This does not mean that you have a CR immediately, but if you see a partial response you can continue in the hope that a CR comes later.”
When asked about duration of PD-1 treatment in patients who have relapsed or do not respond on HPI, Prof Hauschild rationalised: “The duration of treatment is not even defined for melanoma, so typically you treat with a goal to reach a CR, and a CR within 12 months is fine. My impression is that in CSCC, responses are extremely fast, so after just one infusion (i.e., within 3 weeks) we see a response.” In many patients, a response can be seen as quickly as within 8–12 weeks, but in other patients the response may take longer. Prof Hauschild considered that “this is very attractive because it can work so fast in some patients”.
What about Patients with Nonmelanoma Skin Cancer Who Are Not Eligible for Hedgehog Pathway Inhibitors and Programmed Cell Death Receptor 1 Inhibitors?
Prof Hauschild explained there is no age limit for the use of HPI and PD-1 inhibitors, so in principle every patient can be treated. However, organ transplant recipients are at risk of organ rejection with PD-1 inhibitor use, and this treatment gap is challenging. He clarified that there are few patients with HPI-refractory BCC and that these are mostly treated in clinical trials; for patients who progress on HPI, clinical trials are the only option, or PD-1 is given off-label.
SCREENING AND PREVENTION: DOES EARLY DETECTION IMPROVE NONMELANOMA SKIN CANCER PROGNOSIS?
Prof Hauschild outlined that the primary prevention of NMSC is avoidance of sun exposure and the secondary prevention is early detection through skin cancer screening. In Germany, skin cancer screening is an option every 2 years for everyone aged over 35 years, with some insurance companies supporting screening from age 20 years. This has highlighted an increased incidence of NMSC and melanoma, with mainly early cases being detected. In principle, although this has not been evaluated, screening could lead to decreased mortality from melanoma and reduced morbidity and treatment costs in NMSC.
Prof Hauschild described that “the more advanced cases of NMSC are typically treated surgically as inpatients in hospital, which is an expensive setting, whereas the less advanced cases are treated in the ambulatory setting, which is 10 times cheaper than hospital treatment (e.g., €240 EUR versus €2,800 EUR for conventional surgery).” He listed the advantages of screening as avoidance of hospital referrals, treating with smaller margins, and decreased morbidity; however, he acknowledged that the impact of screening is difficult to evaluate because there is no prospective setting in which screened and nonscreened patients are compared.
FUTURE PROSPECTS AND CONCLUSIONS
Dr Algarra considered that there are effective biological treatments now available and clear clinical reasons to offer these treatments to patients; however, only a few skin cancer specialists are experienced in the use of these new treatments. There is a great need for education and dissemination of objective scientific information about these new therapies, mainly among dermatologists, plastic surgeons, and medical and radiation oncologists. It is also very important “to define carefully the agents approved and the right way to use them.” Dr Algarra continued: “We need to approach these patients [with NMSC] with realism as well as with an open mind, considering the activity of these new agents is remarkable and they are going to have a real impact on their lives. We also need to consider that these agents may have side effects and are expensive, so the fine tuning is mandatory.”
Prof Hauschild suggested the best available agents should be given as soon as possible in the first line; however, patient access to a centre of excellence where the drugs are administered may be a problem and the broad education and active collaboration of healthcare professionals in the field are needed. He noted that following the 2019 approval of cemiplimab, the FDA has approved the PD-1 inhibitor pembrolizumab for CSCC, and future areas of research include combining PD-1 inhibitors with EGFR inhibitors. In terms of the future in NMSC, Prof Hauschild concluded: “Second line is very difficult, it looks much better than years ago, but you can always do better… and the PD-1 inhibitors are making a big difference.”
Dr Algarra concluded that these new biological treatments offer a real hope of increasing the quality and length of life for patients who previously had limited choices, as well as an opening for the future development of basic and translational research into the field of NMSC.
The insights of the key opinion leaders in this article clearly show that PD-1 inhibitors are changing and improving the treatment landscape for NMSC by providing alternative strategies for patients who previously had limited or no treatment options.
Biographies
Prof Dr Axel Hauschild
Professor of Dermatology, University of Kiel (UKSH), Kiel, Germany
Prof Hauschild is Head of the Skin Cancer Working Group at the University Hospital Schleswig-Holstein, Campus Kiel, Germany. Prof Hauschild’s main clinical interests are the diagnosis and treatment of melanoma and nonmelanoma skin cancer. He has been the principal investigator of more than 100 Phase I–III clinical trials on melanoma, cutaneous lymphomas, and epithelial skin cancers. Prof Hauschild’s scientific career was honoured with the German Skin Cancer Award and the German Cancer Award. He is the past president of the German Dermatologic Cooperative Oncology Group (DeCOG), and a board member of the European Association of Dermato-Oncology (EADO) and Melanoma World Society (MWS). Prof Hauschild was the congress president of the 8th World Congress on Melanoma in Hamburg (2013) and is the designated president of the 10th World Congress on Melanoma in April 2021 in Rome, Italy. He has been invited as a speaker to more than 700 conferences across the world. Prof Hauschild has published over 420 articles in peer-reviewed journals.
Dr Salvador Martín Algarra
Medical Oncology, Clinica Universidad de Navarra, Pamplona, Spain
Dr Algarra is a consultant of medical oncology at the Clinica Universidad de Navarra (CUN) and a professor of oncology at the Medical School of the University of Navarra in Pamplona, Spain. He has been a member of the Directive Board of the CUN, President of its Educational Board, Co-Director of the Cell Therapy Area of the University of Navarra and Director of the Department of Oncology over two periods, as well as one of the founders and the past President of the Spanish Melanoma Group (Grupo Español Multidisciplinar de Melanoma, GEM). Dr Algarra’s main areas of interest have been the therapeutic development of immunotherapy and targeted therapies in oncology, mainly in melanoma, sarcoma, and rare tumours. He is involved in other areas of clinical oncology as well as in translational research on immunology and biomarkers on solid tumours. His research work in these fields has been published in international journals.








