Speaker: Meteb Al-Foheidi1,2
1. King Saud Bin Abdulaziz University for Health Science, National Guard Health Affairs, Jeddah, Saudi Arabia
2. Saudi Oncology Society (SOS), Riyadh, Saudi Arabia
Disclosure: Prof Al-Foheidi has declared no conflicts of interest.
Acknowledgements: Medical writing assistance was provided by Stefan Amisten, Amisten Consulting, Epsom, UK.
Support: The symposium and the publication of this article were funded by Eli Lilly and Company. The views and opinions expressed are those of the authors and not necessarily of Eli Lilly and Company.
Citation: EMJ Oncol. 2020;8[Suppl 4]:2-7.
Meeting Summary
The 3rd Breast Cancer Virtual Masterclass Symposium took place between 23rd and 25th July 2020 and was hosted by the Saudi Oncology Society (SOS). Prof Al-Foheidi, chairman of the SOS Board of Directors, welcomed 300 medical, radiation, and surgical oncologists; pathologists; radiologists; pharmacists; medical fellows; nurses; and students to this annual masterclass, which, because of the coronavirus disease (COVID-19) pandemic, was offered as a virtual meeting. The annual masterclass provided a knowledge exchange platform for multidisciplinary teams involved in breast cancer care, with the aim of addressing knowledge gaps and improving breast cancer care in Saudi Arabia. The 3-day symposium included a range of presentations focussed on the detection, diagnosis, and management of breast cancer, divided over five sessions and 18 presentations, as well as an Eli Lilly and Company-sponsored satellite symposium. Topics discussed during the masterclass included early breast cancer detection; surgical interventions such as mastectomy, breast-conserving surgery, and lymph node biopsies; novel methods for breast radiotherapy; systemic treatment options for different breast cancer subtypes; the diagnosis and management of triple-negative breast cancer; new treatment options for subtypes of human epidermal growth factor receptor-2 (HER2)-positive tumour types; access to new therapies; genetic testing of breast cancer; improvements in genetic counselling; next-generation sequencing for the identification of the most suitable treatment pathways; palliative care in breast cancer management; and strategies to preserve fertility of young females with breast cancer.
This article discusses a presentation by Prof Al-Foheidi on a MONARCH 2 subgroup analysis of patients receiving abemaciclib plus fulvestrant as first- and second-line therapy for hormone receptor-positive (HR+), HER2-negative advanced breast cancer.1
MONARCH 2: Subgroup Analysis of Patients Receiving Abemaciclib Plus Fulvestrant as First- and Second-Line Therapy for HR+, HER2-Negative Advanced Breast Cancer
Professor Meteb Al-Foheidi
More than 70% of patients diagnosed with metastatic breast cancer present with oestrogen-HR+ disease and are therefore candidates for endocrine therapy. Although initially effective, the benefit of endocrine therapy diminishes as resistance develops.2-4 Oestrogen receptor-induced proliferation is dependent on cyclin D, which is highly expressed in 50% of breast cancer tumours.5-8 Direct inhibition of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6, respectively) diminishes breast cancer cell growth by disrupting the oestrogen receptor-induced proliferation pathway, but continuous inhibition of CDK4 and CDK6 is required for sustained growth arrest with initiation of apoptosis or senescence.9,10
Abemaciclib is an oral, potent, and selective small-molecule inhibitor of CDK4 and CDK6, administered twice daily on a continuous schedule.11-14 Abemaciclib induces G1-phase cell-cycle arrest and abrogation of cell growth through inhibition of CDK4- and CDK6-mediated phosphorylation of the retinoblastoma tumour-suppressor protein.13
Clinical activity of abemaciclib in HR+ metastatic breast cancer as monotherapy and in combination with fulvestrant was first demonstrated in a Phase I study.12 Next, abemaciclib was evaluated in the Phase II MONARCH 1 study; abemaciclib was investigated as a single agent (200 mg given orally, twice daily on a continuous schedule) in patients with hormone-refractory HR+ and HER2-negative metastatic breast cancer.11
The subsequent study, MONARCH 215 (N=669), was a Phase III trial comparing the safety and efficacy of abemaciclib plus fulvestrant, a competitive oestrogen receptor antagonist,16 versus placebo plus fulvestrant in females with HR+ and HER2-negative advanced breast cancer (i.e., defined as inoperable locally advanced or metastatic breast cancer) who progressed while receiving endocrine therapy.17 The MONARCH 2 study enrolled patients who were endocrine resistant and who received study therapy as either first- or second-line treatment for advanced breast cancer. Compared with fulvestrant plus placebo, 150 mg abemaciclib twice daily plus fulvestrant was found to be effective. It significantly improved progression-free survival (PFS), overall survival (OS), and the objective response rate in this subgroup, regardless of menopausal status. Treatment with abemaciclib twice daily plus fulvestrant also had a tolerable safety profile.17,18
The MONARCH 2 study also demonstrated a significant benefit of abemaciclib plus fulvestrant compared with placebo plus fulvestrant in time to second disease progression (PFS2), time to chemotherapy (TTC), and chemotherapy-free survival (CFS).18 Numerically more pronounced, PFS and OS benefit were consistent across all protocol-specified subgroups, including patients with visceral disease and primary endocrine resistance, which are disease factors considered to have a less favourable prognosis.17,18
Subgroup Analysis Results
The aim of this MONARCH 2 subgroup analysis was to report efficacy outcomes for subgroups receiving abemaciclib plus fulvestrant or placebo plus fulvestrant as either first- or second-line therapy.1 The first-line subgroup comprised patients who received abemaciclib plus fulvestrant or placebo plus fulvestrant as first-line treatment for metastatic breast cancer, meaning that their most recent line of endocrine therapy was in the (neo)adjuvant setting. The second-line subgroup comprised patients receiving either abemaciclib plus fulvestrant or placebo plus fulvestrant as second-line therapy for metastatic disease, i.e., patients whose most recent line of endocrine therapy was in the metastatic setting.
Exploratory subgroup analyses were conducted in the intention-to-treat population, stratified by first- versus second-line treatment allocations. PFS was defined as the time from randomisation to objective progression, or death if earlier, and OS was defined as the time from randomisation to death from any cause. Delay in subsequent progression or chemotherapy was investigated through PFS2, time from randomisation to the discontinuation of first subsequent post-discontinuation therapy or death, if earlier; TTC, time from randomisation to initiation of first post-discontinuation chemotherapy, censoring patients who died prior to initiation of chemotherapy; and CFS, time from randomisation to the initiation of first post-discontinuation chemotherapy or death, if earlier. Hazard ratios (HR) were estimated using Cox models, with a test of subgroup interactions with each treatment performed.
At data cut-off (20th June 2019), the first-line therapy group consisted of 265 patients receiving abemaciclib plus fulvestrant, and 133 patients receiving placebo plus fulvestrant. The second-line therapy group included 170 patients receiving abemaciclib plus fulvestrant, and 86 patients receiving placebo plus fulvestrant.1 Baseline characteristics for the first- and second-line subgroups are presented in Table 1.
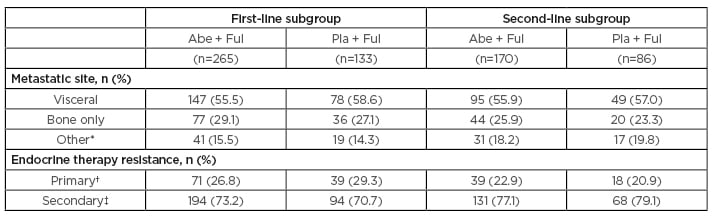
Table 1: Baseline characteristics for first- and second-line subgroups.
*Other soft tissue sites (in the presence/absence of bone metastases).
†In the adjuvant setting, recurrence within the first 2 years of adjuvant endocrine therapy while on endocrine therapy; in the locally advanced or metastatic setting, progression within the first 6 months of initiating first-line endocrine therapy while on endocrine therapy.
‡Patients receiving prior endocrine therapy who do not meet the definition of primary clinical resistance.
Abe: abemaciclib; Ful: fulvestrant; Pla: placebo.
PFS was more favourable for patients treated with abemaciclib plus fulvestrant (median first-line: 15.45 months; median second-line: 17.39 months) compared to patients treated with placebo plus fulvestrant (median first-line: 11.24 months; median second-line: 7.36 months) in both the first- and second-line subgroups (first-line subgroup HR: 0.573 [95% confidence interval (CI): 0.451–0.727]; second-line subgroup HR: 0.478 [95% CI: 0.357–0.639]), and no significant differences between the two treatment subgroups were found (interaction: p=0.341) (Figure 1A).
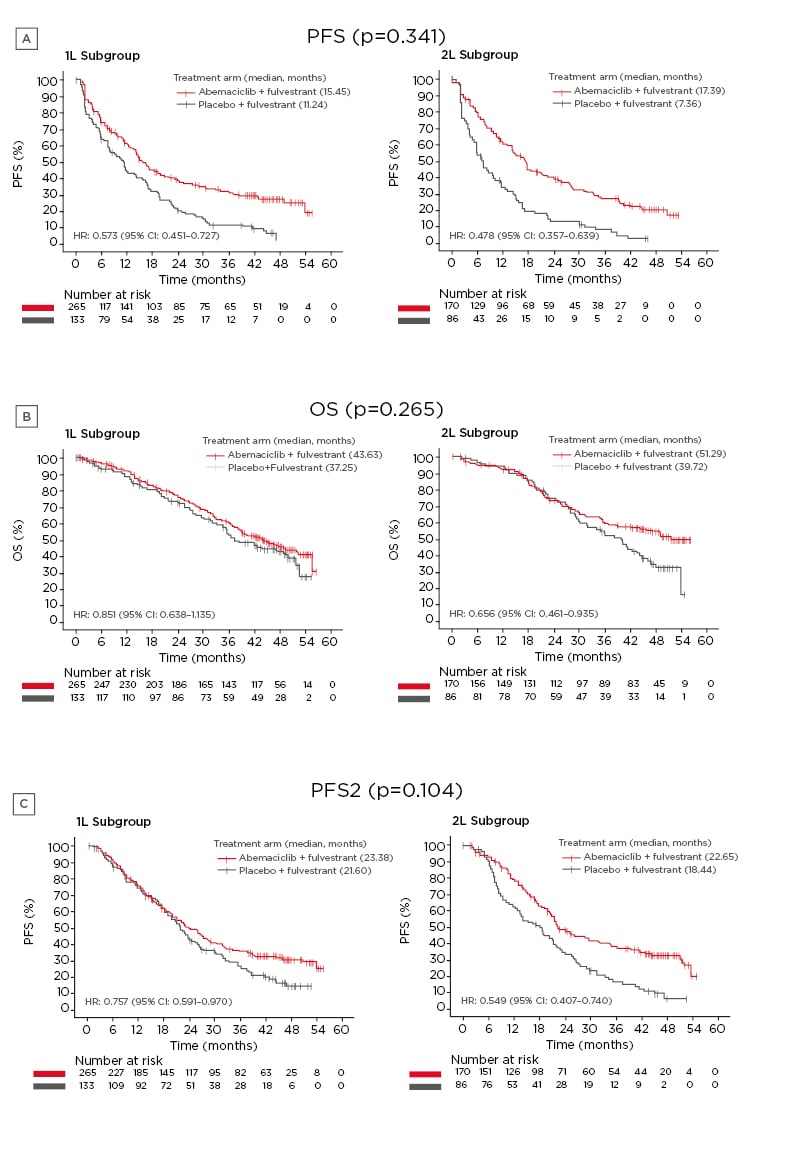
Figure 1: Effects of treatment with either abemaciclib plus fulvestrant or placebo plus fulvestrant in the first- or second-line subgroups.
A) PFS; B) OS; C) PFS2.
CFS: chemotherapy-free survival; CI: confidence interval; HR: hazard ratio; OS: overall survival; PFS: progression-free survival; PFS2: time to second disease progression; TTC: time to chemotherapy; 1L: first-line; 2L: second-line.
Adapted with permission from Neven et al.1
Similarly, OS was higher for abemaciclib plus fulvestrant-treated patients (median first-line: 43.63 months; median second-line: 51.29 months) compared to placebo plus fulvestrant treated patients (median first-line: 37.25 months; median second-line: 39.72 months) in both the first- and second-line subgroups (first-line subgroup HR: 0.851 [95% CI: 0.638–1.135]; second-line subgroup HR: 0.656 [95% CI: 0.461– 0.935]), and no significant differences were found between the two treatment subgroups (interaction: p=0.265) (Figure 1B).
PFS2 was reported after a longer time period for patients treated with abemaciclib plus fulvestrant (median first-line: 23.38 months; median second-line: 22.65 months) compared to patients treated with placebo plus fulvestrant (median first-line: 21.60 months; median second-line: 18.44 months) for both the first- and second-line subgroups (first-line subgroup HR: 0.757 [95% CI: 0.591–0.970]; second-line subgroup HR: 0.549 [95% CI: 0.407–0.740]). As with PFS and OS, no significant differences were found between the first- and second-line treatment subgroups (interaction: p=0.104) (Figure 1C).
A longer delay in TTC was observed for patients treated with abemaciclib plus fulvestrant (median first-line: 45.63 months; median second-line: 51.48 months) compared to placebo plus fulvestrant (median first-line: 27.65 months; median second-line: 12.92 months) for both the first- and second-line subgroups (first-line subgroup HR: 0.815 [95% CI: 0.607–1.095]; second-line subgroup HR: 0.435 [95% CI: 0.311–0.609]). A significantly more favourable outcome was seen for patients in the second-line subgroup compared to patients in the first-line subgroup (interaction: p=0.006).
Similar results were also obtained for CFS, where a better outcome was seen in patients treated with abemaciclib plus fulvestrant (median first-line: 25.74 months; median second-line: 24.56 months) compared to patients treated with placebo plus fulvestrant (median first-line: 22.85 months; median second-line: 12.20 months) for both the first- and second-line subgroups (first-line subgroup HR: 0.808 [95% CI: 0.628–1.039]; second-line subgroup HR: 0.463 [95% CI: 0.343–0.625]). A significantly more favourable outcome was seen for patients in the second-line subgroup compared to patients in the first-line subgroup (interaction: p=0.005).
The first- and second-line subgroups were also analysed based on stratification factors within each subgroup, i.e., nature of disease (visceral, bone only, or other) and sensitivity to endocrine therapy (primary or secondary resistance). For PFS, the most pronounced treatment effects of abemaciclib plus fulvestrant compared to placebo plus fulvestrant within the nature of disease stratification were observed in patients with visceral disease (first-line subgroup HR: 0.535 [95% CI: 0.392–0.731]; second-line subgroup HR: 0.393 [95% CI: 0.269–0.574]). In the sensitivity-to-endocrine-therapy stratification, the biggest impact on PFS was seen in patients with primary resistance to endocrine therapy in the first-line subgroup (HR: 0.401; 95% CI: 0.256–0.628) and in patients with secondary resistance to endocrine therapy in the second-line subgroup (HR: 0.430; 95% CI: 0.307–0.602).
For OS, the most pronounced treatment effects of abemaciclib plus fulvestrant compared to placebo plus fulvestrant within the nature-of-disease stratification were observed in patients with visceral disease (first-line subgroup HR: 0.819 [95% CI: 0.573 1.169]; second-line subgroup HR: 0.514 [95% CI: 0.326–0.811]). In the sensitivity-to-endocrine therapy stratification, the biggest impact on OS was seen in patients with primary resistance to endocrine therapy in the first-line subgroup (HR: 0.583; 95% CI: 0.351–0.970) and in patients with secondary resistance to endocrine therapy in the second-line subgroup (HR: 0.609; 95% CI: 0.410–0.906).
Conclusion
Consistent with the ITT population in MONARCH 2, benefits in PFS and OS were observed across both the first- and second-line treatment subgroups. In first-line subgroup patients, the largest numerical effects in PFS and OS were noted in patients with primary endocrine resistance and visceral disease. In second-line patients, numerical benefits in PFS and OS were observed across primary and secondary endocrine resistance, with numerically more pronounced effects observed in visceral disease. The OS benefit was numerically more pronounced in the second-line subgroup compared to the first-line subgroup, which warrants further OS follow-up.
Prolongation of TTC, CFS, and PFS2 was observed in both the first- and second-line subgroups. Prolongation of TTC and CFS was statistically significantly greater in the second-line subgroup. However, it cannot be ruled out that this result may have been driven by the large differences observed in the placebo arm medians. PFS2 was anchored to discontinuation of the first subsequent line of post-discontinuation therapy regardless of therapy type and was thus more robust.
In conclusion, the statistically significant benefits that have been previously reported in the MONARCH 2 study were also observed across the first- and second-line patients in this subgroup analysis in females with HR+ and HER2-negative advanced breast cancer. In first-line patients, treatment with abemaciclib plus fulvestrant resulted in improvements in PFS and OS, with the most pronounced effects noted in patients with less favourable prognostic factors.

![EMJ Oncology 8 [Supplement 4] 2020 Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/12/EMJ-Oncology-8-Supplement-4-2020-Feature-Image-940x563.jpg)






