Erratum: Standard of Care for the Treatment of Endometrial Cancer: Change Is Afoot
Original citation: EMJ Oncol. 2023; DOI/10.33590/emjoncol/10308081. https://doi.org/10.33590/emjoncol/10308081.
Date correction published: 01.09.23
This infographic was originally published on 17.08.2023. Since then, an erratum has been made. “Dostarlimab 1000 mg Q6W for up to 3 years” originally read “Dostarlimab 100 mg Q6W for up to 3 years”. “Naïve to systemic therapy or had been treated with systemic therapy and had recurred or progressed ≥6 months after completion of treatment” originally read “no previous chemotherapy for EC; Previous adjuvant chemotherapy allowed if completed ≥12 mo ago”. “‡ dMMR status (yes or no); Prior chemotherapy (yes or no); ECOG PS (0 or 1 vs. 2)” originally read “‡ dMMR status (yes or no); Prior chemotherapy (yes or no)”. This has now been updated.
EMJ apologises for the error and any inconvenience caused.
![]()
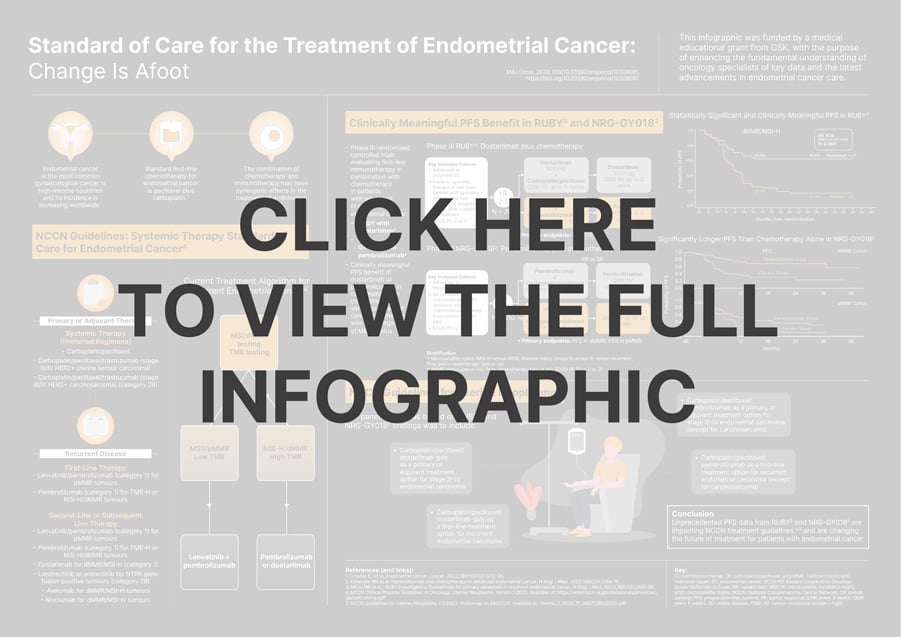
This infographic was funded by a medical educational grant from GSK, with the purpose of enhancing the fundamental understanding of oncology specialists of key data and the latest advancements in endometrial cancer care.
![]()
Explore this infographic for insight on the current National Comprehensive Cancer Network (NCCN) guidelines on the standard of care for systemic therapy in endometrial cancer, including the treatment algorithm for recurrent cases.
The infographic provides a concise overview of the interim findings from Phase III clinical trials, presented at the Society of Gynecologic Oncology (SGO) Annual Meeting 2023. These trials delve into the potential efficacy of combining dostarlimab with chemotherapy for addressing primary advanced or recurrent endometrial cancer (RUBY), along with the prospects of pembrolizumab plus chemotherapy for the management of advanced endometrial cancer (NRG-GY018). Moreover, this visual resource serves as an important update by shedding light on the latest NCCN guidelines for uterine neoplasms.