Abstract
Ovarian cancer is the seventh most common cancer in women and the most lethal gynaecological malignancy. The latest breakthrough in the management of newly diagnosed advanced ovarian cancer has been therapy with the approved poly(ADP-ribose) polymerase (PARP) inhibitors, olaparib, rucaparib, and niraparib. A subgroup analysis from SOLO1 indicated patients with newly diagnosed advanced ovarian cancer achieve substantial benefit from maintenance olaparib regardless of baseline surgery outcome, response to chemotherapy, or BRCA-mutation type. 5-year follow-up data from SOLO1 showed the benefit derived from 2 years of maintenance olaparib was sustained beyond the end of treatment, with almost half of patients on olaparib progression-free at 5 years. Significant improvements in objective response rate and progression-free survival (PFS) were also reported with olaparib in SOLO3 in patients with germline BRCA-mutated, platinum-sensitive, relapsed ovarian cancer. Real-world data indicated PFS and overall survival benefits with olaparib were comparable with results from clinical trials. The significant benefit with rucaparib in the ARIEL3 primary analysis in patients with platinum-sensitive advanced ovarian cancer was also shown in exploratory analyses of patient-centred outcomes in all predefined cohorts, across age subgroups, and over a median follow-up of >2 years in terms of clinically meaningful delay in starting subsequent therapy and lasting clinical benefit. Maintenance niraparib significantly improved PFS in patients with BRCA-mutated advanced ovarian cancer in a post hoc analysis of PRIMA/ENGOT-OV26/GOG-3012 and reduced the risk of disease progression or death by almost three-quarters in patients with platinum-sensitive recurrent ovarian cancer in NORA. PARP inhibitor maintenance treatment of advanced ovarian cancer is associated with long-term efficacy and prolonged PFS compared with placebo in patients with newly diagnosed disease following a complete or partial response to first-, second-, or later-line platinum-based chemotherapy.
INTRODUCTION
Ovarian cancer is the seventh most common cancer in womenand the third most common gynaecological malignancy after cervical and endometrial (uterine) cancers.1-6 Ovarian cancer is the most lethal gynaecologic malignancy globally because of its vague presentation, insidious nature, recurrence, and drug resistance.3,4,7-9 There is no public health screening programme for early detection of ovarian cancer; therefore, most patients with ovarian cancer are diagnosed with advanced (locally advanced or metastatic) disease, which is associated with significant mortality.2,4 A review of global trends in ovarian cancer indicated that just under half of women visited a doctor within 1 month of experiencing symptoms and a quarter waited ≥3 months, with one in four symptomatic women not seeking medical help.10
The incidence of ovarian cancer varies by country, with notable decreases in developed countries and increases in developing countries reported between 1990 and 2017,11 and by ethnicity, with the highest incidence in Caucasian women (12 per 100,000), followed by Hispanic (10.3 per 100,000), Asian (9.2 per 100,000), and African American (0.4 per 100,000) women.1 Various patient factors affect the occurrence of ovarian cancer, with genetic factors (family history, breast-related cancer antigen [BRCA] gene mutations) among the most important.1 As in other diseases, factors including poverty and poor access to healthcare may impact the outcome of ovarian cancer.12
The global incidence of ovarian cancer is expected to rise by 55% to 371,000 cases per year by 2035, newly diagnosed patients with advanced ovarian cancer are at high risk of relapse, current 5-year survival rates are 30–50%, and 15% of women with ovarian cancer die within 2 months of diagnosis; therefore, urgent action is required to improve survival and quality of life in women with this disease.10,13
First-line therapy with a combination of debulking surgery and platinum-based chemotherapy has been the standard of care for decades for women with newly diagnosed advanced ovarian cancer.14-16 The latest breakthrough in the management of newly diagnosed advanced ovarian cancer has been therapy with poly(ADP-ribose) polymerase (PARP) inhibitors.14,17 This article discusses the results published in 2020 and early 2021 from key clinical trials of PARP inhibitors in patients with advanced ovarian cancer in terms of long-term efficacy and survival rates.
PARP INHIBITORS
Homologous recombination and base excision repair are two of the major DNA repair pathways. The proteins encoded by BRCA genes and PARP enzymes are involved in homologous recombination and base excision repair, respectively.18 PARP inhibitors are a group of pharmacological inhibitors of PARP enzymes, which have essential roles in cellular processes, including the regulation of transcription, apoptosis, and the DNA damage response.19 Inhibition of PARP in damaged cells, such as ovarian cancer cells, prevents the DNA repair process and results in disruption of cellular homeostasis and cell death.
BRCA1 and BRCA2 genes play a vital role in cell repair.20 Germline mutations in BRCA1 and BRCA2 account for a large proportion of inherited breast and ovarian cancers.20 Both genes are involved in DNA repair by homologous recombination and are thought to be important in maintaining genomic stability.20 Research into PARP inhibitors was first focused on cancers associated with faulty BRCA genes.21 Tumours with homologous recombination deficiency (HRD), including those in BRCA-mutation carriers, are sensitive to base excision repair blockade via PARP inhibitors.18 Approximately 50% of ovarian carcinomas present HRD and these tumours are more sensitive to platinum and PARP inhibitor therapies.22 Defects in one or both BRCA1 and BRCA2 genes and the resulting deficiency in BRCA1 and BRCA2 proteins induce profound cellular sensitivity to the inhibition of PARP activity.23 HRD and platinum sensitivity are therefore prospective biomarkers for predicting the response to PARP inhibitors in ovarian cancers.24
PARP inhibitors were the first approved cancer drugs that specifically targeted the DNA damage response in BRCA1/2-mutated breast and ovarian cancers.19 PARP inhibitors have transformed the management of advanced ovarian cancer.17,25-27
Three PARP inhibitors have been approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for ovarian cancer:9,28 olaparib (LynparzaÒ; AstraZeneca, Cambridge, UK);29 rucaparib (RubracaÒ; Clovis Oncology, Boulder, Colorado, USA);30 and niraparib (Zejula; GlaxoSmithKline, Brentford, UK) (Table 1).31-34 Veliparib (ABT-888; AbbVie, Chicago, Illinois, USA) is in the late stage of clinical development.31,35
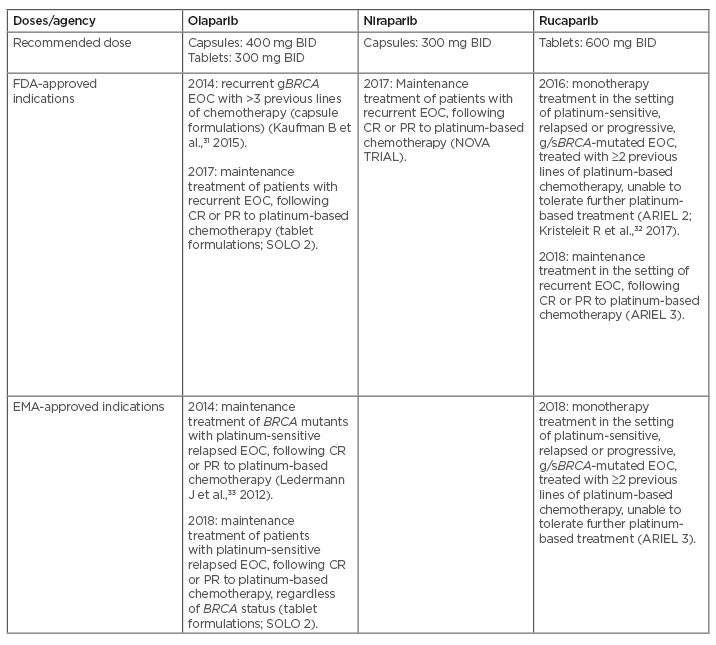
Table 1: FDA/EMA-approved indications for PARP inhibitors in advanced ovarian cancer based on the results of Phase II/III studies.
BID: twice daily; BRCA: breast-related cancer antigen gene; CR: complete response; FDA: U.S. Food and Drug Administration; EMA: European Medicines Agency; EOC: epithelial ovarian cancer; gBRCA: germline BRCA mutation; g/sBRCA: germline/somatic BRCA mutation; PARP: poly(ADP-ribose) polymerase; PR: partial response.
Adapted from Boussios et al.34
Talazoparib (TalzennaÒ; Pfizer, New York City, New York, USA), which has been approved by the FDA and EMA for patients with deleterious or suspected deleterious germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer, is under clinical investigation for ovarian cancer treatment.36,37
OLAPARIB
SOLO1: Benefit of Olaparib Seen Across Subgroups and Maintained for 5 Years
SOLO138,39 was a randomised, double-blind, placebo-controlled, Phase III trial of olaparib as maintenance therapy in patients with newly diagnosed, advanced, high-grade serous or endometrioid ovarian cancer, primary peritoneal cancer, or fallopian tube cancer (or a combination thereof) with a mutation in BRCA1 and BRCA2 who had a complete (CR) or partial (PR) clinical response after first-line platinum-based chemotherapy. A total of 391 patients were randomly assigned (2:1) to receive olaparib tablets (300 mg twice daily; 260 patients) or placebo (131 patients) for up to 2 years or until disease progression.38
In the primary analysis, after a median follow-up of 41 months, patients in the olaparib group derived significant progression-free survival (PFS; primary endpoint) benefit versus placebo (median not reached versus 13.8 months; hazard ratio [HR]: 0.30; 95% confidence interval [CI]: 0.23–0.41; p<0.001).14,38 These results concur with those of SOLO240,41 in patients with platinum-sensitive, relapsed advanced ovarian cancer with a BRCA1/2 mutation who had received at least two lines of chemotherapy, in which median PFS was significantly longer with olaparib than placebo (19.1 versus 5.5 months; HR: 0.30; 95% CI: 0.22–0.41; p<0.0001).
PFS subgroup analyses of SOLO1 showed the risk of disease progression or death was reduced with olaparib compared with placebo by 69% (HR: 0.31; 95% CI: 0.21–0.46) and 63% (HR: 0.37; 95% CI: 0.24–0.58) in patients undergoing upfront or interval surgery, respectively; 56% (HR: 0.44; 95% CI: 0.25–0.77) and 67% (HR: 0.33; 95% CI: 0.23–0.46) in patients with residual or no gross residual disease after surgery, respectively; 66% (HR: 0.34; 95% CI: 0.24–0.47) and 69% (HR: 0.31; 95% CI: 0.18–0.52) in patients with clinical CR or PR at baseline, respectively; and 59% (HR: 0.41; 95% CI: 0.30–0.56) and 80% (HR: 0.20; 95% CI: 0.10–0.37) in patients with a BRCA1 or BRCA2 mutation, respectively.42 These results indicate patients with newly diagnosed advanced ovarian cancer achieve substantial benefit from maintenance olaparib treatment regardless of baseline surgery outcome, response to chemotherapy, or BRCA-mutation type.42
A separate cohort of the SOLO1 study in China investigated the efficacy and safety of maintenance olaparib within the Chinese population (44 patients received olaparib and 20 received placebo).43 Olaparib reduced the risk of disease progression or death by 54% compared with placebo (HR: 0.46; 95% Cl: 0.23–0.97) and median PFS was not reached with olaparib versus 9.3 months with placebo.43 These results support the use of olaparib in Chinese patients with newly diagnosed advanced ovarian cancer who have a BRCA mutation and are in CR or PR after platinum-based chemotherapy.43
As presented at the European Society for Medical Oncology (ESMO) Virtual Congress 2020,13,44 5-year follow-up showed that the benefit of olaparib versus placebo seen in the primary analysis was maintained, with median PFS 56.0 versus 13.8 months (HR: 0.33; 95% CI: 0.25–0.43) after a median of 4.8 and 5.0 years’ follow-up, respectively (Table 2).13,14 Furthermore, risk of disease recurrence or death was reduced by 63% in patients who were in CR at baseline.13,44
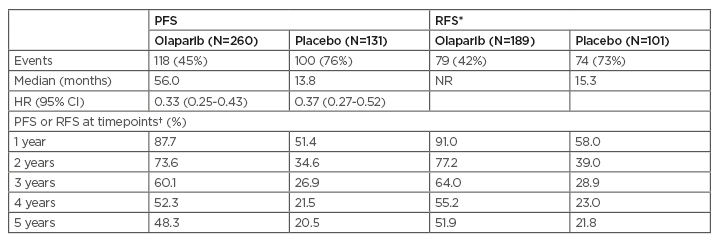
Table 2: Five-year follow-up of olaparib versus placebo.
*Patients had CR at baseline based on electronic case report form data.
†Kaplan–Meier estimates.
The safety profile of olaparib was consistent with previous observations. No new cases of myelodysplastic syndrome of acute myeloid leukaemia were reported and incidence of new primary malignancies remained balanced between arms (olaparib: 7/260 [3%]; placebo: 5/130 [4%]).
CI: confidence interval; CR: complete response; HR: hazard ratio; NR: not reached; PFS: progression-free survival; RFS: relapse-free survival; y: years.
Adapted from Banerjee et al.13
The benefit derived from 2 years of maintenance olaparib was sustained beyond the end of treatment and at 5 years after the start of treatment 48.3% of patients on olaparib were progression-free versus 20.5% on placebo. Furthermore, >50% of patients in CR after first-line platinum-based chemotherapy remained free from relapse 5 years later. No new safety signals were observed in this study.13,44
SOLO3: Significant Improvements in Objective Response Rate and Progression-Free Survival with Olaparib Compared with Non-platinum Chemotherapy in Germline BRCA-Mutated, Platinum-Sensitive Relapsed Ovarian Cancer
SOLO345,46 was a randomised, open-label, Phase III trial of olaparib tablets versus non-platinum chemotherapy in patients with germline BRCA-mutated, platinum-sensitive, relapsed ovarian cancer who had received at least two prior lines of platinum-based chemotherapy. A total of 266 patients were randomly assigned (2:1) to olaparib 300 mg twice daily (178 patients) or physician’s choice single-agent non-platinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan; 88 patients).45
In patients with measurable disease, objective response rate (primary endpoint) was statistically significantly higher with olaparib than with chemotherapy (72.2% versus 51.4%, respectively; odds ratio: 2.53; 95% CI: 1.40–4.58; p=0.002).45 PFS also significantly favoured olaparib versus chemotherapy (HR: 0.62; 95% CI: 0.43–0.91; p=0.013; median 13.4 versus 9.2 months, respectively).45 Adverse events were consistent with the established safety profiles of olaparib and chemotherapy.
Real-World Benefit of Olaparib Comparable with That in Clinical Trials
As most patients with ovarian cancer have advanced disease at diagnosis, understanding how treatments for advanced disease work in real-world settings is necessary to provide optimal care for these patients. In a systematic review of real-world studies of PARP inhibitors (five assessing olaparib, one assessing PARP inhibitors as a composite), median PFS and median overall survival for olaparib were 12.7–15.6 and 30.9–35.4 months, respectively, and therefore slightly higher but comparable with results from randomised controlled trials.47
Further real-world data presented at the ESMO Virtual Congress 202048,49 showed that with an extended follow-up, efficacy and toxicity of olaparib in a real-world cohort of patients48 were consistent with findings observed in SOLO2,40 and that olaparib was well tolerated with no new safety signals observed.49
RUCAPARIB
ARIEL3: Clinical Benefit of Rucaparib Shown Across Age Subgroups and After >2 Years’ Follow-Up
ARIEL350,51 was a randomised, double-blind, placebo-controlled, Phase III trial to evaluate the efficacy of rucaparib after response to second-line or later platinum-based chemotherapy in patients with platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma, who had achieved CR or PR to their last platinum-based regimen. A total of 564 patients were randomly assigned (2:1) to receive oral rucaparib 600 mg twice daily (375 patients) or placebo (189 patients) in continuous 28-day cycles until disease progression, death, or other reason for discontinuation.50
In the primary analysis, median PFS in patients with a BRCA-mutated carcinoma was 16.6 versus 5.4 months in the rucaparib and placebo groups, respectively (HR: 0.23; 95% CI: 0.16–0.34; p<0.0001); in patients with HRD carcinoma, it was 13.6 versus 5.4 months (HR: 0.32; 95% CI: 0.24–0.42; p<0.0001), and in the intention-to-treat (ITT) population, it was 10.8 versus 5.4 months (HR: 0.36; 95% CI: 0.30–0.45; p<0.0001).50 The most common treatment-emergent adverse events of Grade 3 or higher were reported in 209 (56%) versus 28 (15%) patients in the rucaparib and placebo groups, respectively, with anaemia or decreased haemoglobin concentration the most common events.50
A post hoc exploratory analysis of patient-centred outcomes in ARIEL3 showed significantly longer quality-adjusted progression-free survival (QA-PFS) and quality-adjusted time without symptoms or toxicity (Q-TWiST) with rucaparib versus placebo in all predefined cohorts.52 The difference in mean QA-PFS (rucaparib versus placebo) was as follows: ITT population, 6.28 months (95% CI: 4.85–7.47); BRCA-mutated cohort, 9.37 months (95% CI: 6.65–11.85); HRD cohort, 7.93 months (95% CI: 5.93–9.53); and BRCA wild-type/loss of heterozygosity low patient subgroup, 2.71 months (95% CI: 0.31–4.44). The difference in mean Q-TWiST (rucaparib versus placebo) was 6.88 months (95% CI: 5.71–8.23), 9.73 months (95% CI: 7.10–11.94), 8.11 months (95% CI: 6.36–9.49), and 3.35 months (95% CI: 1.66–5.4) in the ITT population, BRCA-mutated cohort, HRD cohort, and BRCA wild-type/loss of heterozygosity low patient subgroup, respectively.52
A further post hoc exploratory analysis to investigate the impact of age on the clinical utility of rucaparib in ARIEL3 showed median PFS was significantly longer with rucaparib than placebo in patients aged <65 years (11.1 versus 5.4 months; HR: 0.33; 95% CI: 0.25–0.43; p<0.0001) and 65–74 years (8.3 versus 5.3 months; HR: 0.43; 95% CI: 0.29–0.63; p<0.0001) and numerically longer in patients aged ≥75 years (9.2 versus 5.5 months; HR: 0.47; 95% CI: 0.16–1.35; p=0.1593).53 QA-PFS and Q-TWiST were significantly longer with rucaparib than placebo across all age subgroups.53 Safety of rucaparib was generally similar across the age subgroups.
The significant benefit with rucaparib versus placebo across all primary analysis groups in ARIEL350 was also observed in prespecified exploratory analyses over a median follow-up of 28.1 months in terms of clinically meaningful delay in starting subsequent therapy and lasting clinical benefit.54 In the ITT population in the rucaparib and placebo groups, respectively, median chemotherapy-free interval was 14.3 versus 8.8 months (HR: 0.43; 95% CI: 0.35–0.53; p<0.0001); median time to start of first subsequent therapy was 12.4 versus 7.2 months (HR: 0.43; 95% CI: 0.35–0.52; p<0.0001); median time to disease progression on subsequent therapy or death was 21.0 versus 16.5 months (HR: 0.66; 95% CI: 0.53–0.82; p=0.0002), and median time to start of second subsequent therapy was 22.4 versus 17.3 months (HR: 0.68; 95% CI: 0.54–0.85; p=0.0007). Chemotherapy-free interval, time to start of first subsequent therapy, time to disease progression on subsequent therapy or death, and time to start of second subsequent therapy were also significantly longer with rucaparib than placebo in the BRCA-mutated and HRD cohorts.54 Updated safety data were consistent with previous reports.
NIRAPARIB
PRIMA/ENGOT-OV26/GOG-3012: Niraparib at Fixed or Individualised Starting Doses Significantly Improved Progression-Free Survival in Patients with BRCA-Mutated Advanced Ovarian Cancer
PRIMA/ENGOT-OV26/GOG-301255,56 was a randomised, double-blind, Phase III trial in which 733 patients with newly diagnosed advanced ovarian cancer were randomly assigned (2:1) to receive niraparib 300 mg once daily (487 patients) or placebo (246 patients) after a response to platinum-based chemotherapy.
In the primary analysis, median PFS was significantly longer with niraparib than with placebo in patients with HRD tumours (21.9 versus 10.4 months, respectively; HR: 0.43; 95% CI: 0.31–0.59; p<0.001) and in the overall population (13.8 versus 8.2 months, respectively; HR: 0.62; 95% CI: 0.50–0.76; p<0.001).
At the 24-month prespecified interim analysis, the rate of overall survival was 84% and 77% in the niraparib and placebo groups, respectively (HR: 0.70; 95% CI: 0.44–1.11). The most common adverse events of Grade 3 or higher were anaemia (31.0% of patients) and thrombocytopenia (28.7%).
A post hoc analysis57 was conducted to assess the efficacy of niraparib in patients with BRCA-mutated advanced ovarian cancer. The trial protocol was amended to incorporate an individualised starting dose (ISD) of 200 mg orally once daily for patients with bodyweight <77 kg or platelet count <150,000 /μL, and a fixed starting dose (FSD) of 300 mg once daily was used in all other patients.
A total of 223 (30%) of the 733 randomised patients had BRCA-mutated tumours. Of those, 144 (65%) received FSD and 79 (35%) received an ISD. PFS benefit with niraparib over placebo was comparable in patients receiving FSD (HR: 0.44; 95% CI: 0.26–0.73) and ISD (HR: 0.29; 95% CI: 0.13–0.67).
NORA: Niraparib Reduced Risk of Disease Progression or Death and Prolonged Progression-Free Survival Compared with Placebo in Patients with Platinum-Sensitive Recurrent Ovarian Cancer Irrespective of BRCA-Mutation Status
NORA58,59 was a randomised, double-blind, placebo-controlled, Phase III trial of maintenance treatment with niraparib in patients with platinum-sensitive recurrent ovarian cancer who had responded to their most recent platinum-containing chemotherapy. A total of 265 patients were randomised 2:1 to receive oral niraparib (300 mg/day; 177 patients) or placebo (88 patients) in 28-day treatment cycles until disease progression, death, unacceptable toxicity, patient withdrawal, or loss to follow-up.58 Following a protocol amendment, patients with bodyweight <77 kg or platelet count <150,000 /μL received 200 mg/day and all other patients received 300 mg/day as an ISD. Sixteen patients were randomised to niraparib 300 mg/day (11 patients) or placebo (5 patients) before the protocol amendment and 249 were randomised using an ISD (14 patients received niraparib 300 mg/day or placebo; 235 patients received niraparib 200 mg/day or placebo).58
Median PFS was significantly longer for patients receiving niraparib versus placebo in the ITT population (18.3 versus 5.4 months, respectively; HR: 0.32; 95% CI: 0.23–0.45; p<0.0001), with niraparib maintenance treatment associated with a 68% reduced risk of disease progression or death.58 The PFS benefit with niraparib was independent of BRCA-mutation status: median PFS was longer for niraparib versus placebo in patients with germline BRCA mutations (not reached versus 5.5 months, respectively; HR: 0.22; 95% CI: 0.12–0.39) and those without germline BRCA mutations (11.1 versus 3.9 months, respectively; HR: 0.40; 95% CI: 0.26–0.61).58 Prespecified subgroup analyses showed that the PFS benefit of niraparib was consistent among all patient subgroups.58 The most common adverse events were decreased neutrophil count (20.3% versus 8.0%) and anaemia (14.7% versus 2.3%).
EXPANSION OF INDICATION AND UPDATED GUIDELINES
Olaparib Indication Expanded for Use in Combination with Bevacizumab
On 8th May 2020, the indication of olaparib monotherapy for first-line maintenance treatment of BRCA-mutated advanced ovarian cancer was expanded to include its use in combination with bevacizumab for first-line maintenance treatment of HRD-positive advanced ovarian cancer and approved by the FDA.28 Both of these approvals (olaparib monotherapy and olaparib in combination with bevacizumab) were based on randomised, double-blind, placebo-controlled trials. Approval for olaparib monotherapy was based on the results of SOLO1;38,39 approval for olaparib in combination with bevacizumab was based on the results of PAOLA-1,60,61 which compared olaparib with bevacizumab versus placebo plus bevacizumab in patients with advanced high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer after first-line, platinum-based chemotherapy.28 These approvals are considered to represent a major advance for the treatment of women with advanced ovarian cancer who are in CR or PR after their initial platinum-based chemotherapy.28
On 21st September 2020, the EMA Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion for olaparib plus bevacizumab as maintenance treatment in adult patients with advanced, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response after first-line platinum-based chemotherapy and whose disease has HRD positivity defined by either a BRCA1/2 mutation or genomic instability.62
PARP Inhibitors in the Recurrent Setting
According to the American Society of Clinical Oncology (ASCO) guideline,63 all patients with newly diagnosed, Stage III–IV epithelial ovarian, tubal, or primary peritoneal cancer whose disease was in CR or PR to first-line, platinum-based chemotherapy with high-grade serous or endometrioid ovarian, tubal, or primary peritoneal cancer should be offered PARP inhibitor maintenance therapy with niraparib. The guideline states that PARP inhibitors are not recommended for use in combination with chemotherapy, other targeted agents, or immune-oncology agents in the recurrent setting outside the context of a clinical trial, and that data to support reuse of PARP inhibitors in any setting are needed.63
Homologous Recombination Deficiency Testing
HRD testing provides an opportunity to optimise the use of PARP inhibitors in patients with ovarian cancer, but methodologies are diverse and clinical application remains controversial.64 ESMO considers currently available HRD tests useful for predicting likely magnitude of benefit from PARP inhibitors but better biomarkers are urgently needed to better identify current homologous recombination proficiency status and stratify ovarian cancer management.64
WHAT IS THE FUTURE FOR PARP INHIBITORS IN ADVANCED OVARIAN CANCER?
Ovarian cancer inevitably acquires resistance to platinum chemotherapy and PARP inhibitors. Acquisition of PARP inhibitor resistance has been shown to be accompanied by increased activity of the cell cycle checkpoint proteins, ataxia telangiectasia mutated and Rad3-related kinase (ATR) and its major downstream effector checkpoint kinase 1 (CHK1), and sensitivity to ATR inhibition.65 Regardless of the mechanisms of resistance, complete and durable therapeutic responses to a PARP inhibitor–ATR inhibitor combination that significantly increased survival were observed in clinically relevant platinum-resistant and acquired PARP inhibitor-resistant patient-derived xenograft models.65 These findings indicate that the PARP inhibitor–ATR inhibitor combination is a promising strategy for ovarian cancers that acquire resistance to PARP inhibitors and platinum.65
Further preclinical research to address acquired resistance to PARP inhibitors in ovarian cancer showed that BET, SRC, and BCL2 family inhibitors were synergistic drug combinations with PARP inhibitors in vitro.66 These findings indicate therapeutic strategies to address PARP inhibitor resistance using approved drugs or agents in clinical development, with the potential for rapid translation to benefit ovarian cancer patients.66
Published data indicate the efficacy of PARP inhibitors may be associated with immunomodulation.67,68 In a proof-of-concept Phase II study, the combination of olaparib and the antiprogrammed cell death ligand-1 (anti-PD-L1) durvalumab showed modest clinical activity in recurrent ovarian cancer, indicating immunomodulatory effects with the combination in these patients.69 This result indicates the potential benefit of combining PARP inhibitors with immunotherapy in patients with ovarian cancer.
CONCLUSION
The use of PARP inhibitors in the management of advanced ovarian cancer has recently emerged as an exciting and effective strategy with the potential to improve outcomes for patients with this disease. This review provides the most recent efficacy and survival data from key clinical trials of the approved PARP inhibitors olaparib, rucaparib, and niraparib. The results provide evidence that use of PARP inhibitors in the maintenance treatment of advanced ovarian cancer is associated with long-term efficacy and PFS in patients with newly diagnosed disease following a CR or PR to first-, second-, or later-line, platinum-based chemotherapy. As most patients with ovarian cancer have advanced disease at diagnosis, understanding how treatments for advanced disease work in real-world settings is necessary to provide optimal care for these patients. Real-world data indicated PFS and overall survival benefits with olaparib were comparable with results from randomised controlled trials. Results from key clinical trials have led to an expansion of indication for olaparib to include combination with bevacizumab as maintenance therapy. Preclinical research showing complete and durable therapeutic responses to a PARP inhibitor–ATR inhibitor combination in PARP-inhibitor resistant patient-derived xenograft models and synergism between BET, SRC, and BCL2 family inhibitors and PARP inhibitors in vitro indicate potential therapeutic strategies to address resistance to PARP inhibitors. Early clinical research combining PARP inhibitors and immunotherapy is also yielding promising results.

![EMJ Oncology 9 [Supplement 4] 2021 Feature Image](https://www.emjreviews.com/wp-content/uploads/2021/06/EMJ-Oncology-9-Supplement-4-2021-Feature-Image-1-940x563.jpg)






