Interviewees: Grace Dy Roswell
Roswell Park Comprehensive Cancer Center, Buffalo, New York, USA
Disclosure: Dy has received honoraria/consulting fees from AstraZeneca, Eli Lilly, Amgen, and Mirati Therapeutics. Dy did not receive financial compensation for this piece of work.
Acknowledgements: This article was authored by Eleanor Roberts, Beeline Science Communications Ltd, London, UK.
Support: The publication of this article is fully funded by Sanofi.
Disclaimer: The opinions expressed in this article belong solely to the named interviewee.
Citation: EMJ. 2022;7[3]:52-59. DOI/10.33590/emj/10113934. https://doi.org/10.33590/emj/10113934.
Interview Summary
Despite therapeutic advances, the prognosis of non-small cell lung cancers (NSCLC) is still very poor, especially when first diagnosed at later stages involving metastases. NSCLC classification can be aided by identifying genetic, molecular, and histological subtypes that are important biomarkers in treatment selection. The majority of targeted therapies are now first-line treatment options for eligible patients with advanced stages of NSCLC. Here they have been shown to improve overall survival (OS) and progression free survival (PFS). Such treatments include those aimed at driver mutations in NSCLC, such as the genes for EGFR and ALK, and immune checkpoint inhibitors such as those targeting programmed death protein 1 or its ligand (programmed death ligand 1 [PD-L1]). In antibody-drug conjugates (ADC), cytotoxic payloads are conjugated to monoclonal antibodies (mAb) that deliver the drug to tumour cells expressing the corresponding target antigen. While there are still no ADCs specifically approved for NSCLC by the U.S. Food and Drugs Administration (FDA), several agents have shown promise and are being investigated as therapy in NSCLC. Emerging biomarkers as targets for ADCs with potential relevance in the treatment of NSCLC include products of the genes CEACAM5, TROP2, HER2, and c-MET. Herein, this interview provides an overview of biomarkers and targeted therapies, with a discussion with Grace Dy, Roswell Park Comprehensive Cancer Center, Buffalo, New York, USA, on their potential clinical utility.INTRODUCTION
Surgical resection is a major treatment approach in early-stage NSCLC, along with chemoradiation for locally advanced disease.1 For patients with locally advanced or metastatic NSCLC, selection of therapy for NSCLC depends on several factors, including disease stage, pattern of local and/or metastatic spread, patient performance status, and comorbidities, along with biomarkers such as histological, mutation profile, and protein expression markers.
To better understand how these biomarkers and drugs could be utilised in clinical practice, EMJ discussed the success of current biomarker-based therapies, unmet needs for patients with NSCLC, the use of novel biomarkers in NSCLC, and the potential role of these biomarkers as drug targets, which are being investigated in a number of clinical trials.
THE SUCCESS OF CURRENT STANDARD OF BIOMARKER-BASED CARE FOR PATIENTS WITH NON-SMALL CELL LUNG CANCERS
Progress is being made in management of late stage NSCLC due to development of targeted therapies against various biomarkers due to better knowledge of tumour biology.2 Driver mutations in NSCLC include those involving different genes such as EGFR, ALK, KRAS, etc.2 For patients with newly diagnosed stage IV, relapsed/recurrent advanced NSCLC, and those without actionable oncogenic biomarkers, choice of therapy may include the use of immune checkpoint inhibitors (as monotherapy or in combination with chemotherapy) especially where there are no driver mutations.1 Such immune checkpoint inhibitors include pembrolizumab, nivolumab, cemiplimab, and atezolizumab, which target programmed death protein 1 or its ligand, PD-L1;1,3-5 and ipilimumab, which targets cytotoxic T-lymphocyte-associated protein 4, with use dependent on expression levels.
In the advanced setting, biomarker testing is routinely used at diagnosis to select options for first and subsequent lines of treatment.1 For example, in the UK, National Institute of Health and Care Excellence (NICE) guidelines recommend the use of biomarker testing to detect EGFR tyrosine kinase mutations in all patients with previously untreated, locally advanced or metastatic NSCLC.6 Dy stated that “testing is only carried out when there’s an impact on clinical practice. For example, testing for EGFR mutation status in NSCLC was only applicable in the advanced setting before December 2020 in the USA because that’s the only setting where therapy was shown to be effective. However, we now test for EGFR mutation status in earlier stages of disease as well as in the adjuvant setting in patients who meet criteria for adjuvant therapy with the tyrosine kinase inhibitor (TKI) osimertinib.”
In patients with later stages of NSCLC, therapy with inhibitors against these targets can improve median PFS and/or OS.1 For instance, in people with EGFR-mutated tumours and previously untreated locally advanced or metastatic NSCLC, treatment with osimertinib demonstrated a very high overall response rate of 80%, and a median PFS of 18.9 months in the FLAURA study, along with superior OS compared with patients who receive a first generation EGFR TKI.7,8 In patients with treatment-naïve advanced ALK-positive NSCLC, the ALEX trial of alectinib showed an overall response rate of more than 80.0%, a median PFS of 34.8 months, and superior 5-year OS compared with crizotinib.9 For patients with KRAS p.G12C-mutated advanced NSCLC previously treated with standard therapies, sotorasib has shown a response rate of around 37.0%, with a median PFS of 6.8 months, and 2-year OS of 32.5% in a pooled analysis.10
With results such as these, Dy discussed how biomarker-based treatments that were initially evaluated as second-line therapies have subsequently been approved for first-line therapy.1 A potential exception to this generalisation, she discussed, are situations where the tumour remains sensitive to immunotherapy despite harbouring actionable mutations such as in NSCLC with a KRAS p.G12C mutation. “For those patients,” Dy suggested, “we would still be very comfortable starting with immunotherapy-based treatment because many patients with KRAS mutations can derive prolonged duration of benefit from immunotherapy if there are no additional biomarker alterations to suggest inferior outcomes with immunotherapy such as co-mutation with serine/threonine kinase 11.”11,12
To illustrate, pembrolizumab has been investigated in a series of studies as mono- or combination therapy for patients with metastatic NSCLC. The KEYNOTE 024 trial showed a median OS of 30.0 months with pembrolizumab alone compared with 14.2 months with chemotherapy alone in patients with EGFR/ALK wildtype NSCLC harbouring a PD-L1 tumour proportion score of ≥50%.4 Where participants were included with any PD-L1 tumour proportion score of >1%, OS was 17.7 months in the pembrolizumab group compared with 13.0 months in the chemotherapy group.13 When pembrolizumab was combined with pemetrexed-platinum in non-squamous NSCLC without EGFR/ALK alterations, a median OS of 22.0 months versus 10.7 months with chemotherapy alone was shown,3 with a median OS of 17.1 months with pembrolizumab plus carboplatin and paclitaxel/nab-paclitaxel versus 11.6 months for placebo plus chemotherapy.14 Similar superior OS signals were seen with ipilimumab plus nivolumab-based regimens compared with chemotherapy alone.15 Such results demonstrate why immune checkpoint inhibitor-based regimens are now first-line therapy for later disease stages of NSCLC.1
Brain metastases are common in NSCLC, occurring in up to 40% of patients.16 Dy reported that treatment choice may be influenced by the presence of such metastases, as some therapies have better central nervous system penetrance than others.17 “Many, if not all, of these agents with better brain activity have been adopted as preferred treatment in the first-line setting,” explained Dy. The classic example is osimertinib, which has better central nervous system penetrance compared with early generation TKIs and is now the preferred first-line treatment in the USA.18
NEW BIOMARKERS AND DRUG TARGETS IN NON-SMALL CELL LUNG CANCERS MANAGEMENT
According to Dy, “there remains a need for both new drug targets as well as platforms in testing for drug targets.” Four emerging biomarkers in NSCLC are CEACAM5, TROP2, HER2, and c-MET. Mutation or amplification of these genes, or overexpression of the corresponding proteins, can influence NSCLC prognosis and treatment outcomes.19-22
Carcinoembryonic antigen cell adhesion molecule 5 (CEACAM5) is a protein involved in cell adhesion, migration, and anoikis inhibition. This latter property may facilitate tumourigenesis and metastasis and its expression in a variety of cancers has led to it being investigated as a biomarker and therapeutic target in NSCLC.19 Tumour-associated calcium signal transducer 2 is involved in calcium transduction as well as tumour growth, invasion, and neovascularisation. Overexpression is associated with a poor clinical course in people with lung adenocarcinoma.20 Mutation, amplification, and over-expression of the transmembrane glycoprotein receptor gene HER2 is well documented in NSCLC and associated with poorer prognosis and resistance to EGFR-focused TKIs in some patients with NSCLC.21 C-mesenchymal epithelial transition factor, a tyrosine kinase receptor, is involved in cancer cell survival, proliferation, migration, invasion, and angiogenesis and is also associated with EGFR and ALK TKI resistance.22
In the case of CEACAM5, while not all NSCLC-associated tumours express this marker to a high degree (as assessed, for instance, by rating immunohistochemical stain intensity and proportion of positive stained cells), such expression was found in approximately 24% of specimens in one study.23
CEACAM5 expression on circulating tumour cells can also be monitored in the circulation.24 However, explained Dy, “a caveat here is that CEACAM5 is non-specific. Inflammatory conditions, diarrhoea, or smoking can cause it to go up. Nonetheless, it is helpful overall in monitoring trends over time especially in lung cancer patients who are non-smokers, who have elevated CEACAM5 levels prior to therapy.” HER2 mutations are much rarer,21 and for c-MET “it is more complicated,” reported Dy, “as exon 14 mutation, amplification, or protein overexpression may be potentially used as biomarkers depending on the therapeutic platform utilised.”22
However, while these biomarkers are known, Dy explained how, in the clinic, “we don’t routinely test these currently because they’re not necessarily actionable in terms of standard of care therapies. With that being said, for example, when we do find acquired resistance to TKIs such as those mediated by c-MET overexpression, we can now triage patients into MET-based trials.”
THE POTENTIAL ROLE OF EMERGING BIOMARKERS AS DRUG TARGETS IN THE TREATMENT OF PATIENTS WITH NON-SMALL CELL LUNG CANCERS
There is a role for these emerging biomarkers as drug targets, according to Dy. More recently the use of ADCs associated with CEACAM5, TROP2, HER2, and c-MET expression have been investigated for NSCLC. These consist typically of a mAb, such as tusamitamab, sacituzumab, trastuzumab, or telisotuzumab, targeting the tumour-expressed antigen, conjugated to a cytotoxic drug (termed a ‘payload’ or ‘warhead’), such as ravtansine, deruxtecan, or vedotin. Attaching the payload to the mAb in the ADC is made possible through ‘linker’ molecules. The goal of ADCs is to maximise tumour cell kill, while minimising systemic toxicity to healthy cells.25 Examples of these are shown in Table 1.
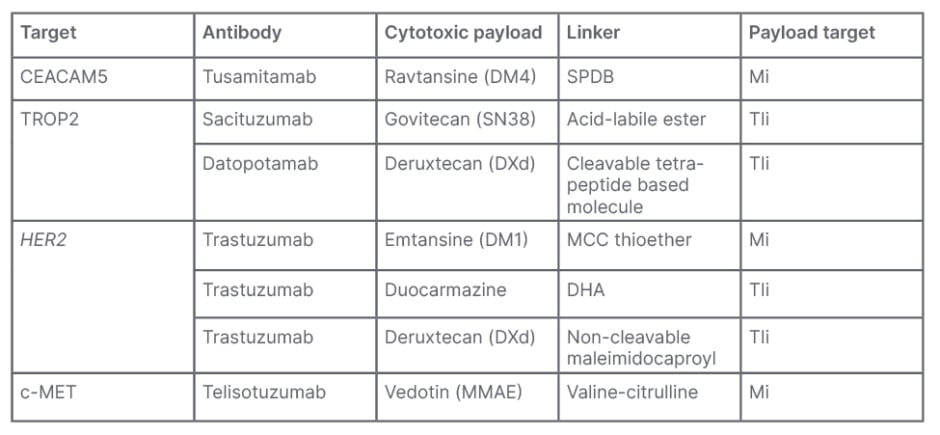
Table 1: Examples of antibody-drug conjugates.25-30
CEACAM5: carcinoembryonic antigen cell adhesion molecule 5; c-MET: c-mesenchymal epithelial transition factor; DHA: duocarmycin-hydroxybenzamide-azaindol; MCC: maleimidomethyl cyclohexane-1-carboxylate; Mi: microtubule inhibitor; MMAE: monomethyl auristatin E; SPDB: succinimidyl 4-(2-pyridyldithio) butyrate; TIi: topoisomerase I inhibitor; TROP2: tumour-associated calcium signal transducer 2.
Notably, Dy explained that “many of the ADCs in development have toxicities that are better tolerated.”25,26 However, she added the caveat that, “of course, the toxicity profile will vary among ADCs, not just because of the target but also because of the differences in payloads that may have unique side effects that oncologists may not be familiar with initially.25,26 But over time, we become more vigilant in monitoring a patient’s symptoms, for example with ocular toxicities associated with maytansinoid DM4.”
Dy also explained how there are many ADC trials exploring combinations with immunotherapy in the first-line setting. “We don’t anticipate ADCs to replace immunotherapies,” she said, “but we are looking at how we complement existing treatments. For instance, do we combine ADC with immunotherapy or targeted therapy first-line? I do foresee that ADCs would have some role, but it takes a while to develop these drugs. We must first prove the therapy’s value before we can routinely implement them as a standard of care. Testing for the relevant biomarkers would certainly be absorbed into practice once we show benefit of these newer therapies.” Other questions she posed that need investigating included: “How do we modify the chemotherapy regimen that is combined with immunotherapy now and do we drop one of the agents to minimise myelosuppression, depending on the payload?”
One issue associated with ADC therapy, highlighted Dy, “is the efficacy of the payload itself and the linker technology that may enable bystander killing effect.” She also highlighted how the level of molecular biomarker expression may not always correspond to how well a drug works. For instance, Dy discussed how, in her experience, the ADC trastuzumab-deruxtecan can work even in patients with HER2 mutated NSCLC despite low HER2 expression, as has been shown in people with advanced breast cancer with low HER2 expression.31 Additional prospective studies are needed to validate and confirm these observations. However, Dy stressed that, “we’re still very enthused because we have all these new ADCs coming into the clinic showing promise in clinical trials. The next phase though is how do we incorporate testing in the setting of limited tissue and biological variations such as intra-tumour heterogeneity.”
CLINICAL UNMET NEEDS FOR PATIENTS WITH NON-SMALL CELL LUNG CANCERS
“The biggest challenge in NSCLC,” discussed Dy, “is the stage at diagnosis as usually patients are diagnosed with an advanced or a metastatic stage not amenable to surgery.” This, she highlighted, necessitates lung cancer screening programmes, as these have demonstrated benefits in terms of early diagnosis and lung cancer-specific survival and mortality outcomes.32
Other challenges involve therapy options at different stages, and the success of such lines of treatment, and how best to treat specific metastases. Dy reported that, “while we’re able to optimise first-line therapies for specific patient populations where targeted therapy or immunotherapy can be superior to chemotherapy, the vast majority of patients, even those eligible for these therapies, will inevitably experience disease progression.” This is potentially due to resistance to targeted therapy or immunotherapy, discussed Dy, who commented that for almost all patients “we have to define what the mechanism of acquired resistance is because sometimes there is a subsequent therapy to address that resistance. Inevitably though,” she continued, “at some point, the evolutionary biology of cancer resistance outpaces our ability to understand things and develop therapies rapidly in real-time in response to these resistance mechanisms.”
Another unmet need Dy discussed was identifying better biomarkers for immunotherapy and the need to improve on immunotherapy treatment outcomes in ‘cold tumours’ (where effector immune cell activity is suppressed) or ‘immune deserts’ (where immune cells are not in the vicinity of the tumour). There is also the problem, she highlighted, that large proportions of patients have tumours that either do not have a targeted therapy option available or are unlikely to respond well to immunotherapy.
While in Dy’s centre they look for biomarker protein overexpression using immunohistochemistry, she explained how challenges arise in the early phases of drug development due to not having standardised methods for, for example, choosing a positive/negative cut-off, defining overexpression, or having specific reagents such as the antibody for protein detection with immunohistochemistry. This, she explained, makes it difficult to rapidly implement certain biomarkers into routine practice.
A further limitation, Dy discussed, is that “tissue biopsies from patients with lung cancer are limited in amount, and it might not be easy to re-biopsy to get more tissue in many situations.” She stressed how “having multiple targeted therapies available will not be useful unless we are able to test for actionable mutations properly and expediently.” With newer biomarkers, Dy explained how they are currently mostly used for academic research. “We have all these newer pathology techniques that are being developed that might actually be tissue-sparing but at this point, deciding when to do testing is still potentially problematic because even the current standard of adequate mutation profiling is still not met for many patients.”
More widely, Dy discussed the problem of general resource variance when it comes to being able to detect, analyse, and treat NSCLC. This is not only between low- versus high-income countries, but even within the same country, for example, metropolitan versus rural areas. Cost was also an issue raised in that while there may be a number of biomarkers that could be used to analyse NSCLC, in many countries only a limited number of biomarkers are currently covered by healthcare and insurance providers. She emphasised that even though current guidelines endorse biomarker testing for newly diagnosed patients, due to deficits in local resources, many with advanced NSCLC “may not get beyond EGFR and ALK testing despite the fact that we now have multiple effective targeted therapies.”33
ONGOING CLINICAL TRIALS OF EMERGING BIOMARKERS
There are several ongoing Phase II and III trials of therapies associated with emerging biomarkers. For instance, in the case of CEACAM5, the CARMEN studies are investigating the use of tusamitamab ravtansine, including in a global Phase III trial where it is being compared with docetaxel (CARMEN-LC03; NCT04154956),34 and a Phase II single arm trial combining tusamitamab ravtansine with ramucirumab to examine efficacy and toxicity (CARMEN-LC04; NCT05245071).35 Also underway are CARMEN-LC05 (NCT04524689), evaluating tusamitamab ravtansine either with pembrolizumab or pembrolizumab and platinum-based chemotherapy with or without pemetrexed,36 and CARMEN-LCO6 (NCT05245071), evaluating tusamitamab ravtansine in participants with negative or moderate CEACAM5 expression and high circulating carcinoembryonic antigens.35
For TROP2 mutations, there are several ADCs in development, including several TROPION trials investigating datopotamab-deruxtecan,37-43 and the EVOKE trials investigating sacituzumab govitecan-hziy (NCT05089734 and NCT05186974).44,45 For HER2 mutations, there are the DESTINY trials investigating trastuzumab deruxtecan (NCT05048797).46 Other HER2 selective agents under investigation include tucatinib (NCT04579380),47 or those that can dually targeted EGFR and HER2, such as BDTX189 (NCT04209465)48 or BAY2927088 (NCT05099172).49
With c-MET mutations, Dy discussed how, aside from ADCs, other antibody-based approaches include selective agents being tested in combination with, for instance, those targeting EGFR mutations. Amivantamab, an EGFR/c-MET bispecific antibody, is approved for treatment in patients with an EGFR exon 20 insertion mutation in the second-line setting.50 It is also being tested in combination with chemotherapy upfront for this patient population in the PAPILLON trial (NCT04538664).51 Amivantamab is additionally being tested in combination with lazertinib as a first-line option (NCT04965090)52 or in combination with chemotherapy after osimertinib failure (NCT04487080)53 in patients with classic EGFR mutation in the MARIPOSA trial.
CONCLUSION
Despite therapeutic advances, the prognosis of NSCLC is still very poor.54 Identification of genetically and molecularly defined NSCLC subtypes has led to development of biomarkers for such and the use of targeted therapies. Many more are also in the clinical trial phase. While use of such biomarkers and therapies has led to gains in NSCLC in terms of PFS and OS, current limitations include access to the means to analyse tumours, to the tissue needed to carry out a number of analyses, and to the therapies themselves.
References
- PDQ Adult Treatment Editorial Board. Non-Small Cell Lung Cancer Treatment (PDQ®): Health Professional Version. 2022. Available at: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Last accessed: 31 August 2022.
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12(2):175-80.
- Gadgeel S et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-17.
- Reck M et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537-46.
- Bossageon M et al. First-line treatment of advanced non-small-cell lung cancer with immune-checkpoint inhibitors: new combinations and long-term data. BioDrugs. 2022;36(2):137-51.
- National Institute for Health and Care Excellence (NICE). EGFR-TK mutation testing in adults with locally advanced or metastatic non-small-cell lung cancer. 2013. Available at: https://www.nice.org.uk/guidance/dg9/resources/egfrtk-mutation-testing-in-adults-with-locally-advanced-or-metastatic-nonsmallcell-lung-cancer-29280700357. Last accessed: 31 August 2022.
- Soria JC et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-25.
- Ramalingam SS et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2019;382(1):41-50.
- Mok T et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056-64.
- Dy GK et al. Long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100. Abstract CT003. American Association for Cancer Research (AACR) Annual Meeting. April 8-13, 2022.
- Skoulidis F et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021;384(25):2371-81.
- Sun L et al. Association between KRAS variant status and outcomes with first-line immune checkpoint inhibitor-based therapy in patients with advanced non-small-cell lung cancer. JAMA Oncol. 2021;7(6):937-9.
- Mok TSK et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-30.
- Paz-Ares L et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657-69.
- Paz-Ares LG et al. First-line nivolumab plus ipilimumab in advanced nsclc: 4-year outcomes from the randomized, open-label, phase 3 checkmate 227 part 1 trial. J Thorac Oncol. 2022;17(2):289-308.
- Nishino M et al. Brain metastases in oncogene-driven non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(Suppl 3):S298-307.
- Page S et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer. 2020;132:187-98.
- Ballard P et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130-40.
- Zhang X et al. CEACAM5 stimulates the progression of non-small-cell lung cancer by promoting cell proliferation and migration. J Int Med Res. 2020;48(9):300060520959478.
- Mito R et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol Int. 2020;70(5):287-94.
- Ren S et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open. 2022;7(1):100395.
- Friedlaender A et al. The METeoric rise of MET in lung cancer. Cancer. 2020;126(22):4826-37.
- Adam J et al. Therapeutic targets in non-small cell lung cancer: preclinical and human studies of carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) expression and its associated molecular landscape. Abstract 19P. ESMO Immuno-Oncology Congress, 8-11 December, 2021.
- Papadaki MA et al. Assessment of the efficacy and clinical utility of different circulating tumor cell (CTC) detection assays in patients with chemotherapy-naïve advanced or metastatic non-small cell lung cancer (NSCLC). Int J Mol Sci. Int J Mol Sci. 2021;22(2):925.
- Marks S, Naidoo J. Antibody drug conjugates in non-small cell lung cancer: An emerging therapeutic approach. Lung Cancer. 2022;163:59-68.
- Desai A et al. Antibody-drug conjugates: a promising novel therapeutic approach in lung cancer. Lung Cancer. 2022;163:96-106.
- Sheyi R, de la Torre BG, Albericio F. Linkers: an assurance for controlled delivery of antibody-drug conjugate. Pharmaceutics. 2022;14(2):396.
- Banerji U et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124-35.
- Gazzah A et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol. 2022;33(4):416-25.
- Okajima D et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20(12):2329-40.
- Modi S et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20.
- Henschke CI et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763-71.
- Robert NJ et al. Biomarker tissue journey among patients (pts) with untreated metastatic non-small cell lung cancer (mNSCLC) in the U.S. Oncology Network community practices. J. Clin. Oncol. 2021;39(Suppl 15):9004.
- Sanofi. SAR408701 versus docetaxel in previously treated, carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) positive metastatic non-squamous non-small cell lung cancer patients (CARMEN-LC03). NCT04154956. https://clinicaltrials.gov/ct2/show/NCT04154956.
- Sanofi. Tusamitamab ravtansine in NSQ NSCLC participants with negative or moderate CEACAM5 expression tumors and high circulating CEA (CARMEN-LC06). NCT05245071. https://clinicaltrials.gov/ct2/show/NCT05245071.
- Sanofi. Tusamitamab ravtansine (SAR408701) in combination with pembrolizumab and tusamitamab ravtansine (SAR408701) in combination with pembrolizumab and platinum-based chemotherapy with or without pemetrexed in patients with NSQ NSCLC (CARMEN-LC05) (CARMEN-LC05). NCT04524689. https://clinicaltrials.gov/ct2/show/NCT04524689.
- Daiichi Sankyo, Inc. Study of Dato-DXd plus pembrolizumab vs pembrolizumab alone in the first-line treatment of subjects with advanced or metastatic NSCLC without actionable genomic alterations (TROPION-Lung08). NCT05215340. https://clinicaltrials.gov/ct2/show/NCT05215340.
- Daiichi Sankyo, Inc. First-in-human study of DS-1062a for advanced solid tumors (TROPION-PanTumor01). NCT03401385. https://clinicaltrials.gov/ct2/show/NCT03401385.
- Daiichi Sankyo, Inc. Study of DS-1062a versus docetaxel in previously treated advanced or metastatic non-small cell lung cancer with or without actionable genomic alterations (TROPION-LUNG01). NCT04656652. https://clinicaltrials.gov/ct2/show/NCT04656652.
- Daiichi Sankyo, Inc. Study of DS-1062a in advanced or metastatic non-small cell lung cancer with actionable genomic alterations (TROPION-Lung05). NCT04484142. https://clinicaltrials.gov/ct2/show/NCT04484142.
- Daiichi Sankyo, Inc. A Study of Dato-DXd in Chinese patients with advanced non-small cell lung cancer, triple-negative breast cancer and other solid tumors (TROPION-PanTumor02). NCT05460273. https://clinicaltrials.gov/ct2/show/NCT05460273.
- AstraZeneca. Datopotamab Deruxtecan (Dato-DXd) in combination with durvalumab with or without carboplatin in subjects with advanced or metastatic non-small cell lung cancer (TROPION-Lung04). NCT04612751. https://clinicaltrials.gov/ct2/show/NCT04612751.
- Daiichi Sankyo, Inc. Datopotamab Deruxtecan (Dato-DXd) in combination with pembrolizumab with or without platinum chemotherapy in subjects with advanced or metastatic non-small cell lung cancer (TROPION-Lung02). NCT04526691. https://clinicaltrials.gov/ct2/show/NCT04526691.
- Gilead Sciences. Study of sacituzumab govitecan-hziy (sg) versus docetaxel in participants with advanced or metastatic non-small cell lung cancer (NSCLC) with f37progression on or after platinum-based chemotherapy and anti-programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) immunotherapy (EVOKE-01). NCT05089734. https://clinicaltrials.gov/ct2/show/NCT05089734.
- Gilead Sciences. Study of sacituzumab govitecan-hziy combinations in first-line treatment of participants with advanced or metastatic non-small-cell lung cancer (NSCLC) without actionable genomic alterations (EVOKE-02). NCT05186974. https://clinicaltrials.gov/ct2/show/NCT05186974.
- AstraZeneca. A study to investigate the efficacy and safety of trastuzumab deruxtecan as the first treatment option for unresectable, locally advanced/metastatic non-small cell lung cancer with HER2 mutations. NCT05048797. https://clinicaltrials.gov/ct2/show/NCT05048797.
- Seagen Inc. Basket study of tucatinib and trastuzumab in solid tumors with HER2 alterations. NCT04579380. https://clinicaltrials.gov/ct2/show/NCT04579380.
- Black Diamond Therapeutics, Inc. A study of BDTX-189, an orally available allosteric ErbB inhibitor, in patients with advanced solid tumors. (MasterKey-01). NCT04209465. https://clinicaltrials.gov/ct2/show/NCT04209465.
- Bayer. First in human study of BAY2927088 in participants who have advanced non-small cell lung cancer (NSCLC) with mutations in the genes of epidermal growth factor receptor (EGFR) and/or Human Epidermal Growth Factor Receptor 2 (HER2). NCT05099172. https://clinicaltrials.gov/ct2/show/NCT05099172.
- National Institute for Health and Care Excellence (NICE). Amivantamab. 2022. Available at: https://bnf.nice.org.uk/drugs/amivantamab/. Last accessed: 31 August 2022.
- Janssen Research & Development, LLC. A Study of combination amivantamab and carboplatin-pemetrexed therapy, compared with carboplatin-pemetrexed, in participants with advanced or metastatic non-small cell lung cancer characterized by epidermal growth factor receptor (EGFR) exon 20 insertions (PAPILLON). NCT04538664. https://clinicaltrials.gov/ct2/show/NCT04538664.
- Memorial Sloan Kettering Cancer Center. A study of amivantamab and lazertinib in people with non-small cell lung cancer (NSCLC). NCT04965090. https://clinicaltrials.gov/ct2/show/NCT04965090.
- Janssen Research & Development, LLC. A Study of Amivantamab and Lazertinib Combination Therapy Versus Osimertinib in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (MARIPOSA). NCT04487080. https://clinicaltrials.gov/ct2/show/NCT04487080.
- American Cancer Society. Cancer facts & figures 2022. 2022. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html#:~:text=Estimated%20numbers%20of%20new%20cancer,factors%2C%20early%20detection%2C%20and%20treatment. Last accessed: 31 August 2022.








