Abstract
The prognosis of mantle cell lymphoma (MCL) has improved rapidly over recent years with the evolution of new management strategies. The disease, once considered fatal, has now become more of a chronic illness, with recurrent relapses that can be managed with a variety of treatment modalities, such as chemoimmunotherapy, stem cell transplantation, and novel targeted therapies. Several treatment options are already available for young, fit patients with newly diagnosed MCL, while many newer agents are being tested in relapsed/refractory MCL. The need for more effective treatment strategies in the elderly population is being addressed by numerous ongoing studies. With the advent of newer treatment modalities with more efficacy and less toxicity, it is now necessary to re-evaluate the way MCL is managed. This paper provides a comprehensive review of emerging, novel agents for the treatment of MCL.
INTRODUCTION
Mantle cell lymphoma (MCL), a distinct and aggressive form of B cell lymphoma, represents about 7% of all lymphomas in Europe and the USA. The median age at diagnosis is 60 years, with a male predominance (2:1). Patients generally present with advanced-stage (Stage III–IV) disease, extensive lymphadenopathy, blood and bone marrow involvement, and splenomegaly. Some present with pancytopenia or extensive leukocytosis (leukaemic presentation). Extranodal sites, such as the gastrointestinal tract, are also frequently involved.1 The pathological hallmark of MCL is the expression of the cyclin D1 protein, which occurs as a result of aberrant expression of the B Cell Lymphoma 1 gene (BCL1). A small number of MCL cases express cyclin D2 or D3 instead of cyclin D1. Additionally, some MCL cases have other acquired alterations, such as abnormalities in TP53 or the deletion of the INK4a/ARF locus on chromosome 9p21. Cyclin D1-negative cases are very rare and may express SOX11 (SRY-Box 11), which is highly specific for MCL.2
The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms subdivided MCL into indolent variants (leukaemic, non-nodal, and in situ MCL) and classical MCL.3 The two subtypes have variable clinical courses. A minority of patients with indolent disease may survive many years without treatment, whereas, in most patients, it behaves more aggressively. There is no clear demarcation between indolent and aggressive variants and treatment depends on the patient’s prediagnosis health status, performance status, disease burden, and age, as well as other prognostic factors. The current standard treatment is chemoimmunotherapy with or without autologous stem cell transplantation (ASCT). Although initial therapy can achieve high overall response rates (ORR), most patients eventually succumb to their disease. Novel therapeutic agents targeting specific abnormalities have shown efficacy in relapsed/refractory disease and are now being tested as frontline treatment. In this review, the authors explore the role of current treatment modalities in the context of developing new targeted therapies for MCL.
RISK ASSESSMENT
Risk stratification in MCL combines clinical, laboratory, radiological, and molecular findings. The recently formulated MCL Prognostic Index (MIPI) and its simplified version, which take into account independent prognostic factors, such as age, performance status, leukocyte count, and lactate dehydrogenase (LDH), have made it easier to stratify MCL patients into low, intermediate, or high-risk groups for treatment purposes. The prognostic factors for shorter overall survival (OS) according to the MIPI are higher age, worse Eastern Cooperative Oncology Group (ECOG) performance status, higher LDH level, and higher white blood cell count at diagnosis. The Ki-67 protein is an independent predictor of outcome and its measurement provides additional discriminatory power to the MIPI.4,5 Some proliferation-associated genes, such as RAN, MYC, SLC29A2, and TNFRSF10B, were identified as prognostic factors in a small study but are yet to be validated by additional studies.6,7 A complex karyotype is associated with decreased progression-free survival (PFS) and aggressive disease in newly diagnosed MCL, and is considered a strong predictor of OS independent of MIPI.8,9
WAIT AND WATCH
Over the past few years, researchers have tried to identify a subgroup of patients who have indolent disease with extended survival. Although specific diagnostic criteria are not available for the recognition of these patients, some clinicopathological studies have identified the non-nodal leukaemic variant with splenomegaly, low Ki-67 proliferation index, lack of SOX11 expression, and hypermutated immunoglobulin heavy chain: a complex karyotype with normal LDH, and β2-microglobulin levels as potential predictors of indolent behaviour.6 These patients usually have a low MIPI score and are asymptomatic. Two separate studies reported by Martin et al.10 and Eve et al.11 in 2009 indicated that these patients could be kept on ‘wait and watch’ for a long period of time without any detrimental effect on outcome. Occasionally, secondary abnormalities, often involving TP53, may occur and lead to very aggressive disease, emphasising the importance of close surveillance in these cases.12
INITIAL MANAGEMENT
Elderly or Low-Risk Mantle Cell Lymphoma Patients
Considering the incurable nature of MCL and the high rate of toxicity associated with currently available dose-intensified regimens, most elderly patients or those with low MIPI scores and/or asymptomatic disease can be managed safely with observation until they become symptomatic. For symptomatic, elderly patients, for whom the intensive treatment strategies are not viable, the choice of therapy includes various nonintensive chemoimmunotherapy regimens, each with different survival benefits and toxicity profiles (Table 1).13-20 Bendamustine plus rituximab (RTX) (BR); RTX, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); R-CHOP followed by RTX maintenance; and consideration for clinical trials are standard options for these patients.
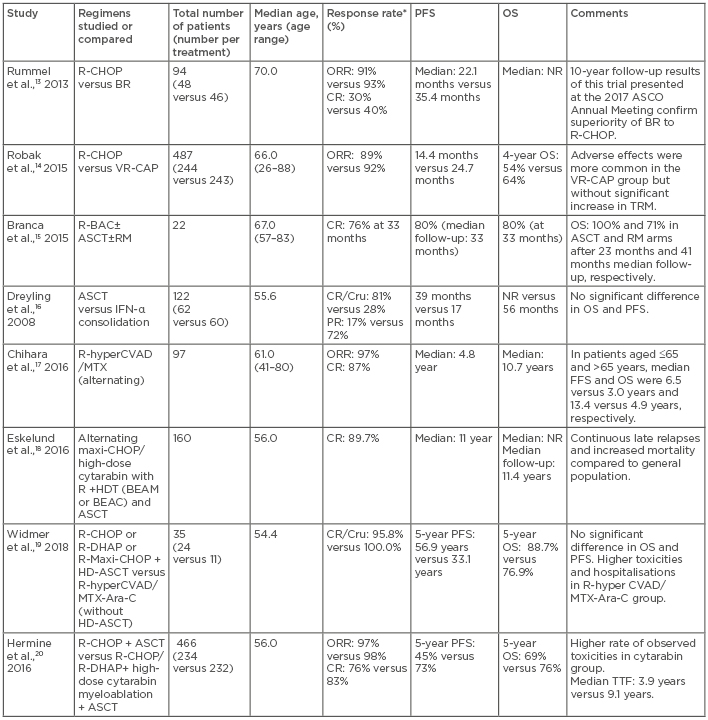
Table 1: Comparison of some of the first-line treatment regimens for mantle cell lymphoma.
*Response rate reflects response after ASCT, as applicable.
Ara C: cytarabin; ASCO: American Society of Clinical Oncology; ASCT: autologous stem cell transplant; BEAM: carmustine (BCNU), etoposide, cytarabine, melphalan; BEAC: BCNU, etoposide, cytarabine, cyclophosphamide; BR: bendamustine, rituximab; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; CR: complete response; Cru: complete response unconfirmed; DHAP: dexamethasone, high-dose cytarabine, cisplatin; FFS: failure-free survival; HDT: high-dose therapy; hyperCVAD: hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; MTX: methotrexate; NR: not reached; OS: overall survival; PFS: progression-free survival; PR: partial response; R: rituximab; R-BAC: rituximab, bendamustine, cytarabin; RM: rituximab maintenance; TBI: total body irradiation; TTF: time to failure; TRM: transplant related mortality; VR-CAP: bortezomib, rituximab, cyclophosphamide, doxorubicin, prednisone.
Young, Fit Symptomatic Patients
Intensive chemoimmunotherapy with or without ASCT remains a cornerstone of MCL treatment in young, fit patients. The MCL Network Phase III trial16 established the superiority of ASCT over INF-α treatment following CHOP in the frontline setting. Molecular remission is considered one of the important predictors of favourable treatment outcome.21,22 Several studies17-20 have evaluated different induction regimens, which can yield complete remission (CR) or negative minimal residual disease before ASCT consolidation. Currently, the standard induction regimens for young, fit patients include intensive chemoimmunotherapy regimens, such as RTX-hyper-cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone (CVAD); methotrexate (MTX); or cytarabine (Ara-C), or a modified Nordic regimen (maxi-CHOP) (alternating with RTX plus high-dose cytarabine), or less intensive regimens (such as R-CHOP) alternating R-CHOP and RTX plus dexamethasone, high-dose cytarabine, and cis-platin (R-DHAP); or BR. In transplant-eligible patients, these regimens are followed by ASCT consolidation in first CR. Results of some studies comparing different treatment strategies in newly diagnosed MCL cases are depicted in Table 1.13-20
AUTOLOGOUS STEM CELL TRANSPLANTATION
ASCT has been tested in various clinical MCL settings and superior outcomes have been reported. In a report by the European MCL Network, 122 patients who responded to initial CHOP-like therapy were randomly assigned to ASCT or two additional cycles of consolidation followed by IFN-α maintenance; the patients receiving ASCT had superior PFS and OS. Similar encouraging results were obtained in other studies,23,24 leading to the establishment of ASCT as a component of frontline therapy for MCL in young, fit patients. Although the treatment-related toxicity and mortality associated with ASCT have always been a cause of concern and hesitation for its use in the elderly population, some recent studies have suggested that ASCT consolidation might be safe, feasible, and worth consideration in selected patients >65 years old.25,26 Yet, poor quality of life, long-term side effects, and late relapses seen in patients who survive long after high-dose therapy and ASCT have compelled scientists to investigate other potentially curative and less toxic regimens. The role of consolidation with ASCT after intensive chemoimmunotherapy is being considered. Various combinations of chemoimmunotherapies and novel agents are being studied, with the aim of replacing ASCT as a frontline therapy in MCL.17,18 The long-term outcome report of a Phase II study investigating RTX-hyper-CVAD alternating with a MTX-Ara-C combination without ASCT in newly diagnosed young MCL patients reported an ORR of 97%, CR of 87%, and median OS and failure-free survival of 13.4 years and 6.5 years, respectively.18 These results, therefore, demonstrated the feasibility of ASCT-free treatment in MCL.
MAINTENANCE THERAPY
Studies have indicated that incorporating a maintenance therapy into the treatment strategy of MCL prolongs remission duration and ultimately survival. The Phase III LyMA trial27 compared maintenance RTX therapy versus observation following treatment with R-DHAP±R-CHOP, high-dose therapy, and ASCT in MCL patients <66 years old and observed superior 4-year OS (89% [RTX] versus 80% [observation]) and PFS (83% [ASCT] versus 64% [observation]; p<0.001) in the RTX maintenance arm. Additionally, long-term follow-up of the randomised European MCL elderly trial,28 which evaluated RTX versus IFN maintenance following initial response to R-CHOP, revealed a 5-year PFS and an OS of 51% versus 22% (p<0.0001) and 79% versus 59% (p=0.002), respectively. Similarly a study comparing RTX-fludarabine and cyclophosphamide (R-FC) with R-CHOP followed by maintenance with either RTX or IFN-α in patients ≥60 years old (median age: 70 years) reported that although CR rates were similar with R-FC and R-CHOP (40% and 34%, respectively), progressive disease was more frequent with R-FC and OS was significantly shorter with R-FC than with R-CHOP (4-year survival rate: 47% versus 62%, respectively). Among patients who responded to R-CHOP, maintenance therapy with RTX significantly improved OS compared with those who received maintenance with INF (4-year survival rate: 87% versus 63%).29 Results of these trials encouraged investigators to explore options for durable maintenance therapy. Nevertheless, not all patients benefit from maintenance therapy, as seen from the results of a subgroup study of the StiL NHL trial.13 The trial compared the effect of RTX maintenance with observation after first-line treatment with BR in patients with previously untreated MCL and found no survival benefit in patients receiving RTX compared to those on observation after 4.5 years follow-up.
MANAGEMENT OF RELAPSED/REFRACTORY MANTLE CELL LYMPHOMA
A watch and wait strategy can be feasible in some relapsed asymptomatic patients who have an indolent course. Once symptoms arise, various treatment options can be considered, including radiotherapy for local relapse, radioimmunotherapy, targeted agents, chemotherapy, and immunomodulatory agents, such as the BR regimen, bortezomib, lenalidomide, or ibrutinib (Table 2).30-34 Though the use of high-dose chemotherapy and ASCT has not demonstrated promising results in the relapsed/refractory setting, results of some studies indicate that in certain patients with long initial responses to salvage therapy, ASCT after second CR can be of benefit.35
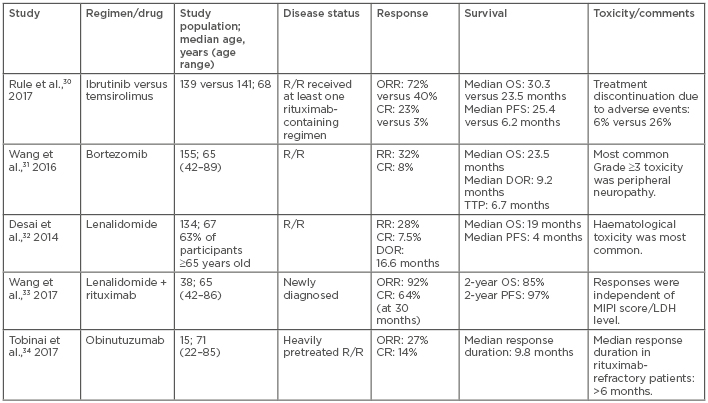
Table 2: Novel agents used in the treatment of mantle cell lymphoma.
CR: complete response; DOR: duration of response; LDH: lactate dehydrogenase; MIPI: Mantle Cell Lymphoma Prognostic Index; NR: not reached; ORR: overall response rate; OS: overall survival; PFS: progression-free survival; R/R: relapsed/refractory; RR: response rate; TTP: time to progression.
NOVEL THERAPIES
Burton’s Tyrosine Kinase Inhibitors
The B cell receptor is a surface receptor complex on B cells and signals through the spleen tyrosine kinase, phosphoinositide-3-kinase (PI3K), Burton’s tyrosine kinase (BTK), and protein kinase C beta. These signals lead to NFκB and AKT activation, which promotes survival and proliferation of normal and malignant B cells. Persistent activation of the B cell receptor pathway has been found to be a major contributor to the pathogenesis of MCL and targeting this pathway has been shown to be effective in MCL.36
Ibrutinib is an oral irreversible BTK inhibitor that binds to cysteine 481 in the phosphorylation site of BTK. The results of a Phase II trial demonstrating a 68% ORR, 21% CR, and median PFS of 13.9 months in a study cohort that included heavily pretreated patients and those with high MIPI scores led to the approval of ibrutinib for previously treated MCL patients.37
Real-world data on the efficacy and outcome of ibrutinib are sparse. Results of a study using data from the global Named Patient Program (NPP),38 including 715 patients from 26 countries with a median age of 70 years who received ibrutinib for relapsed/refractory MCL, were presented at the 21st Congress of the European Hematology Association (EHA). These results were similar to those of the Phase III RAY (MCL3001) trial,29 which showed that 52.3% (95% confidence interval: 43.5–60.4) of global patients remain on treatment after 12 years. Another study that analysed pooled data from 370 patients, with a median age of 67 years, who were receiving ibrutinib for their relapsed/refractory MCL, and enrolled across three different studies (PCYC-1104 [n=111], SPARK [n=120], and RAY [n=139]), demonstrated an excellent outcome, with a median duration of follow-up of 41.1 months, CR rate of 26.5%, median PFS of 13.0 months, and median OS of 26.7 months.39 Despite these encouraging results, it has been observed that 30–40% of patients with MCL do not respond to ibrutinib and even among those who respond initially, the majority of patients ultimately develop resistance.40 Other studies have revealed that patients who fail ibrutinib therapy are not likely to respond to salvage chemotherapy and have a poor outcome, with an OS of 2.9 months.41 A Phase I study by Martin et al.42 reported that palbociclib, a selective CDK4/CDK6 inhibitor, can overcome ibrutinib resistance and sensitise MCL cells to ibrutinib in certain patient groups, achieving a better response rate in patients receiving a combination of these drugs compared to ibrutinib alone.
Acalabrutinib, a novel irreversible second-generation BTK inhibitor with a high rate of durable response and favourable safety profile, has recently been approved for use in relapsed/refractory MCL by the U.S. Food and Drug Administration (FDA) following the results of a Phase II, single-arm, multicentre trial.43 The study included 124 patients with relapsed/refractory MCL who had received a median of two previous therapies. All patients received acalabrutinib 100 mg twice a day until disease progression or an unacceptable toxicity level were reached. This resulted in an ORR of 81%, CR of 40%, and a 12-month OS and PFS of 72% and 87%, respectively. In addition, tirabrutinib (ONO/GS-4059), another oral BTK inhibitor, demonstrated a relative response rate of 92% (11 of 12 participants) in patients with a median treatment duration of 40 weeks.44 Other BTK inhibitors thought to be more selective and potent are also being developed and have shown promising results.45
Phosphoinositide-3-Kinase Inhibitors
Idelalisib, an oral potent inhibitor of the ď-isoform of PI3K, has been implicated in the regulation of the activation, proliferation, migration, and survival of B lymphocytes. In a Phase I dose-escalation study46 of idelalisib, which enrolled 40 previously treated (a median of four prior therapies) MCL patients, an ORR of 40% was observed. However, the duration of response and PFS were very short (2.7 months and 3.7 months, respectively).46 In a Phase II safety and efficacy study of copanlisib, a pan-class I PI3K inhibitor, in patients with relapsed/refractory indolent and aggressive lymphomas, including 11 MCL patients, a response was seen in 7 out of the 11 recruited MCL patients (2 CR and 5 partial responses, with an ORR of 63.6%).47 Duvelisib (IPI-145), an oral PI3K inhibitor, has also shown efficacy in mouse models of MCL.31
Bortezomib
Bortezomib is a proteasome inhibitor that induces cell cycle arrest and apoptosis in MCL cells. In addition, it sensitises malignant lymphoid cells to the cytotoxic effects of chemotherapy and glucocorticoids. The PINNACLE trial48 and LYM-300214 study led to the FDA approval of bortezomib for the treatment of relapsed/refractory MCL and as a frontline therapy for MCL, respectively. The combination of bortezomib with various chemotherapeutic agents has been tested previously and in the ongoing trials. The bendamustine, bortezomib, and RTX regimen (BVR) remains the therapeutic pathway of choice. The BVR regimen resulted in an ORR of 71% in relapsed/refractory MCL patients with manageable toxicities in one study,49 and is also being studied in an intergroup randomised Phase III trial as a frontline therapy for older, treatment-naïve MCL patients, with the results awaited.50
Lenalidomide
Lenalidomide is a structural analogue of thalidomide with enhanced immunological and anticancer properties and less severe toxicity. It is an immunomodulator that works through multiple mechanisms, including, but not limited to, direct tumour cytotoxicity; inhibition of angiogenesis; interaction with the tumour microenvironment; modulation of vascular endothelial growth factors; and inhibition of metastasis and cellular proliferation.32 Extensive preclinical and clinical studies (EMERGE)51 led to the FDA approval of lenalidomide for the treatment of MCL patients whose disease progressed or relapsed after two prior therapies (one of them including bortezomib).
Lenalidomide as a single agent is effective in the management of MCL in patients who have progressed, relapsed, or are intolerant or refractory to novel agents, such as ibrutinib.33 The combination of lenalidomide with various agents, such as dexamethasone, bendamustine, temsirolimus, and RTX, has been tested in numerous Phase II and III trials,52 out of which the combination of lenalidomide with RTX has been deemed more effective and less toxic than other drug combinations (Table 2).30-34 In one study, this combination was found to be useful as an initial therapy for MCL, with 80% of patients achieving minimal residual disease-negative CR after 3 years of treatment. This response was associated with improved quality of life and manageable toxicity. The promising results from these studies warrant a head-to-head comparison with standard regimens, particularly in patients who are not eligible for intensive chemotherapy and ASCT. However, lenalidomide-based regimens may impair haematopoietic stem cell collection after prolonged therapy and compromise outcomes of subsequent ASCT in eligible patients. Patients receiving lenalidomide for MCL can experience a tumour flare reaction, a syndrome that presents with painful lymph nodes and/or spleen enlargement and can be accompanied by fever, rash, and clear lymphocytosis.
Temsirolimus and Everolimus
The identification of the involvement of the PI3-kinase/Akt/mTOR pathway in the pathogenesis of MCL led to the investigation of temsirolimus as a possible therapy for MCL. Two separate Phase II trials tested two different doses of temsirolimus: a 250 mg weekly intravenous dose53 and a 25 mg weekly intravenous dose.54 The two trials resulted in an ORR of 38% and 41%, respectively, with dose-dependent haematological toxicities. A subsequent randomised Phase III trial55 comparing temsirolimus in two dosing levels with a regimen of choice, selected by the investigators, showed that 175 mg weekly temsirolimus for 3 weeks followed by 75 mg weekly had an ORR of 22% and a median PFS and OS of 4.8 months and 12.8 months, respectively. These data led to the approval of temsirolimus for use in relapsed MCL in the European Union (EU). A study combining temsirolimus with RTX56 observed an ORR of 59% and a CR of 18.5%, with a median OS of 29.5 months and time to progression of 9.7 months. These results are comparable to the lenalidomide-RTX combination, but the temsirolimus–RTX combination was associated with a higher incidence of severe toxicities. Another mTOR inhibitor, everolimus, has also demonstrated activity in MCL in a Phase IItrial,57 and it is being explored as part of combination regimens alongside other investigational MCL therapies.
Venetoclax
Venetoclax is an oral selective inhibitor of the prosurvival protein BCL2 and restores the apoptotic ability of malignant cells. This is a promising agent showing activity in relapsed/refractory MCL. In an initial Phase I study of relapsed/refractory non-Hodgkin’s lymphoma, the cohort of relapsed/refractory MCL patients (n=28) who had received a median of three previous therapies attained an ORR and a CR of 75% and 21%, respectively, and 1-year OS was 82%, with a median PFS of 14 months. The most common Grade 3–4 toxicity was haematological.58 The combination of venetoclax and ibrutinib was investigated in a Phase II study that included 23 patients with relapsed/refractory MCL, 30% of whom had failed ASCT, while one was a treatment-naïve MCL patient (5%). OR and CR were achieved in 71% and 63% of all patients, respectively, and the estimated PFS and OS was 74% and 81%, respectively, at 8 months.59 A Phase III trial60 comparing a combination of venetoclax and ibrutinib versus ibrutinib and placebo in MCL patients aged ≥18 years is ongoing, with the aim of evaluating dose-limiting toxicities, occurrence of tumour lysis syndrome, and PFS among two study groups.
MISCELLANEOUS AGENTS
Monoclonal Antibodies
RTX is a type I chimeric anti-CD20 antibody that induces cell death primarily through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. RTX, as a single agent or in combination with various chemotherapy regimens, has been extensively tested and used as frontline therapy and maintenance therapy in MCL.15-17,55 However, suboptimal responses and resistance to RTX have remained a challenge. Ofatumumab is a fully human type I anti-CD20 monoclonal antibody that binds to a different epitope of CD20 than RTX, resulting in higher binding affinity and enhanced complement-dependent cytotoxicity.34 Obinutuzumab is a humanised, type II, anti-CD20 monoclonal antibody. In culture and xenograft models, obinutuzumab has demonstrated an improved ability to induce direct cell death, as well as antibody-dependent cellular cytotoxicity, compared with RTX.61 Ofatumumab and obinutuzumab have been approved for use in certain patients with follicular lymphoma and chronic lymphocytic leukaemia, and are being studied in MCL.
Chimeric Antigen Receptor T Cells
In chimeric antigen receptor (CAR) T cell therapy, immune cells are taken from a patient’s bloodstream and are reprogrammed to recognise and attack a specific protein found in cancer cells. The cells are then reintroduced into the patient, allowing the cells to detect and destroy targeted tumour cells. The anti-CD19 CAR T cell product, axicabtagene ciloleucel, has been approved in patients with relapsed/refractory diffuse large B cell lymphoma based on the results of the ZUMA-1 trial.62 Axicabtagene ciloleucel is now being investigated in relapsed/refractory MCL in the ZUMA-2 trial (Table 3).50,63-67 Case reports of anti-CD19 CAR T cells improving the response to chemotherapy in chemoresistant MCL have been reported;68 however, further studies are needed to estimate the potential of anti-CD19 CAR T cell therapy in MCL.
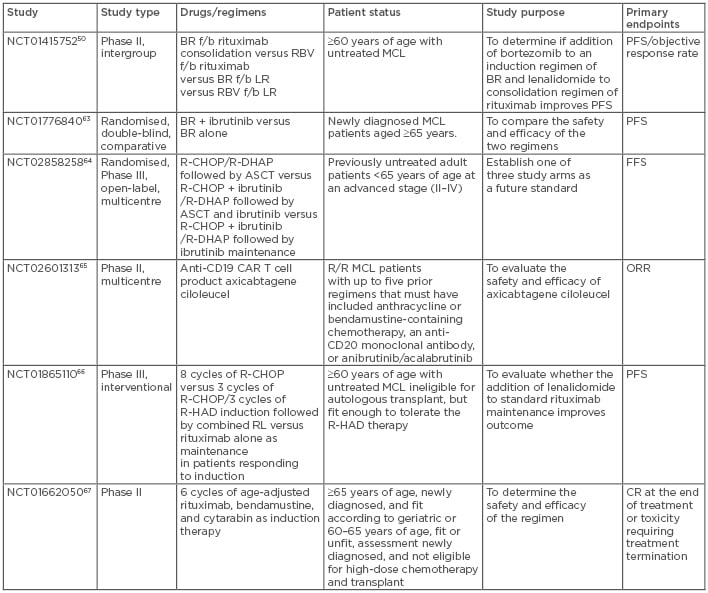
Table 3: Ongoing clinical trials in mantle cell lymphoma.
ASCT: autologous stem cell transplantation; BR: bendamustine, rituximab; CAR: chimeric antigen receptor; CR: complete response; f/b: followed by; FFS: failure-free survival; MCL: mantle cell lymphoma; ORR: overall response rate; PFS: progression-free survival; R/R: relapsed/refractory; R-CHOP: rituximab-cyclophosphamide, doxorubicin, vincristine, prednisone; R-DHAP: rituximab-dexamethasone, high-dose cytarabine, cisplatin; R-HAD: rituximab, high-dose cytarabine, dexamethasone.
Allogeneic Stem Cell Transplant
The potential benefit of allogeneic stem transplantation (alloSCT) is related to the graft-versus-lymphoma effect and the low risk of therapy-related myelodysplastic syndrome/acute myeloid leukaemia. Myeloablative alloSCT is not an option for the majority of MCL patients because of their older age at diagnosis and presence of comorbidities. Multiple study groups have investigated the role of reduced-intensity conditioning alloSCT (RIST) in MCL in a small series and have reported conflicting outcomes.69-72 A retrospective registry analysis of a large cohort of patients (N=324), which included patients who had undergone RIST for MCL from January 2000 to December 2008, was published recently. The study reported a higher toxicity rate and relapse rate of 25% and 40%, respectively, at 1 and 5 years associated with chemo-refractory disease post transplantation (hazard ratio: 0.49; p=0.01) and concluded that RIST cannot be recommended as a routine part of first-line therapy, for which ASCT remains the consolidation procedure of choice.73 Durable remissions have been reported with alloSCT but at the expense of higher treatment-related mortality; hence, this potentially curative procedure should be reserved for highly selected patients, such as those with multiply relapsed or refractory disease.
ONGOING CLINICAL TRIALS AND RESEARCH
There are numerous ongoing studies of patients with MCL. Some studies are evaluating different chemoimmunotherapy novel agent combinations, whereas others are investigating entirely chemotherapy-free regimens in the relapsed/refractory as well as frontline settings (as standalone regimens or as induction regimens before ASCT). Studies of particular significance are listed in Table 3.50,63-67
CONCLUSION
MCL is predominantly a disease of older patients, for whom intensive chemotherapy regimens are often poorly tolerated. Even in younger patients, the long-term side effects of intensive chemotherapy regimens are significant. Chemotherapy-free combination regimens represent a potential novel approach. The recent observation that the negative prognostic impact of TP53 mutations is not observed in patients treated with ibrutinib, lenalidomide, and RTX combination therapy supports the continued investigation of this regimen and similar regimens to formulate a chemotherapy-free and less toxic treatment regimen for MCL patients. Until then, intensive chemoimmunotherapy followed by ASCT, when feasible, remains the best standard of care.








