Abstract
Lung cancer is the most frequently diagnosed cancer type and the leading cause of cancer-related deaths worldwide. According to the last GLOBOCAN estimate of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC), lung cancer accounted for approximately 13% of cancer diagnoses in 2012, and an estimated 1.8 million new lung cancer cases were diagnosed. First-line treatment for Stage IV non-small cell lung cancer (NSCLC) has changed considerably, primarily as a result of a better patient selection on the basis of histology, molecular markers, and innovative treatment approaches.
Recent data have highlighted the advent of immunotherapy as the major shift in treatment of advanced NSCLC. Three checkpoint inhibitors of the programmed death-1–programmed death-ligand 1 interaction, nivolumab, pembrolizumab, and atezolizumab, have already received U.S. Food and Drug Administration (FDA) approval for treatment of advanced NSCLC patients; however, despite impressive treatment responses in many patients who received immunotherapy, a cohort of patients failed to obtain significant results. This review summarises the emerging role of immunotherapy in NSCLC, emphasising the current unanswered questions about predictive biomarkers for treatment response, current treatments, and possible treatment combinations.
INTRODUCTION
The last GLOBOCAN project in 2012 estimated that lung cancer was a leading cause of cancer-related deaths in males worldwide and among females in more developed countries, with an estimated 1.8 million new lung cancer cases diagnosed that year, accounting for approximately 13% of all cancer diagnoses.1 The emergence of immune checkpoint inhibitors has contributed to an improved prognosis for a large proportion of non-small cell lung cancer (NSCLC) patients. In recent months, the programmed death-1 (PD-1)–programmed death-ligand 1 (PD-L1) checkpoint inhibitors, nivolumab, pembrolizumab, and atezolizumab, have received U.S. Food and Drug Administration (FDA) approval for the treatment of advanced NSCLC. The Phase III KEYNOTE-024 clinical trial evidenced superior efficacy and overall survival (OS) of pembrolizumab compared with platinum-doublet chemotherapy in untreated NSCLC patients with high PD-L1 expression (≥50% of tumour cells), promoting immunotherapy approaches as a new standard of care for advanced NSCLC.2-7 This review summarises the emerging role of immunotherapy in NSCLC, highlighting uncertainties regarding predictive biomarkers for treatment response, current treatments, and possible treatment combinations.
IMMUNOTHERAPY IN LUNG CANCER
For a long time, lung cancer was considered a non-immunogenic neoplasm. There have been several randomised clinical trials using the Bacillus Calmette-Guérin (BCG) vaccine, interleukin-2 alone or in combination with other cytokines, or interferon-α alone or in combination with chemotherapy; all showed unfavourable results.8-10 Recent research has confirmed that lung cancer involves genetic and/or epigenetic alterations that can lead to the generation of neoantigens, known as fragments of mutated proteins displayed in the major histocompatibility complexes of tumour cells critical for the anti-tumour immune response.11
PROGRAMMED DEATH-LIGAND 1: PROMISES AND LIMITATIONS
Increasing evidence suggests that the predominant mechanism by which lung cancer cells evade the host’s immunological response is through the expression of PD-L1, also called B7-H1 or CD274.12 PD-1 is an immune-regulatory receptor expressed on the surface of activated T cells, B cells, and natural killer cells. The PD-1–PD-L1 interaction inhibits T cell responses, induces apoptosis of tumour-specific T cells, promotes differentiation of CD4+ T cells into regulatory T cells, and promotes tumour cell resistance.13-15
Clinical trials showed that anti-PD-1 and anti-PD-L1 antibodies produced durable responses in approximately 20% of unselected patients with advanced NSCLC.4,16 It was thought that these receptors would be good candidate biomarkers for selecting patients who were more responsive to immunotherapy; however, not all PD-L1-positive patients are likely to respond to treatment and, more importantly, some patients who test negative for the antibodies may still respond, making it an imperfect biomarker.17
In addition to previous considerations, questions have been raised about the technical aspects of PD-L1 testing; these factors included the specificity of several clones of anti-human PD-L1 antibodies for immunohistochemistry (IHC) and the artefacts that may be derived from different techniques for tissue fixation and antigen retrieval.18 Most of these technical concerns were clarified by IHC assay standardisation but, even with standardised reagents, tissue processing, and test performance, it is difficult to obtain a dichotomous result from a PD-L1 assay since there is no consensus on the level of PD-L1 required to separate positive from negative results.13 In fact, based on KEYNOTE-001 findings, it is still unclear if PD-L1 can be expressed as a continuous measure rather than a binary positive or negative result. Along with these uncertainties, studies have shown varied results, finding that PD-L1 positivity indicated a favourable, unfavourable, or non-existent relationship to prognosis, as well as variable correlations with histology and mutation status in NSCLC and other tumour types.13,19 PD-L1-positive values in different reported series ranged from 1–50%, making it difficult to compare results across different studies.16
To help solve this issue, an industrial–academic collaborative partnership of drug manufacturers and representatives from Dako and Ventana, the FDA, the American Association of Cancer Research (AACR), the American Society of Clinical Oncology (ASCO), and the International Association for the Study of Lung Cancer (IASLC) was formed. The Blueprint PD-L1 IHC Assay Comparison Project was launched to provide information on analytical and clinical comparability of four PD-L1 IHC assays used in clinical trials. In particular, 22C3 (Dako, Carpinteria, California, USA), 28-8 (Dako), SP142 (Ventana Medical Systems, Tucson, Arizona, USA), and SP263 (Ventana Medical Systems) assays were evaluated; 22C3, 28-8, and SP142 were closely aligned on tumour cell staining and were therefore used, whereas SP263 showed consistently fewer stained tumour cells. The authors evidenced that despite similar analytical performance of PD-L1 expression for the three assays, interchanging assays and cut-off limits would lead to misclassification of PD-L1 status for some patients.20
Despite the development and criticisms of these commercial IHC assays, the role of PD-L1 as a predictive biomarker was also confounded by multiple unresolved biological issues, including different expressions in primary versus metastatic biopsies, oncogenic versus induced PD-L1 expression, intratumour heterogeneity, and staining of tumour cells versus immune cells. In particular, PD-L1 can be expressed by both tumour and inflammatory cells within the tumour microenvironment but the relative importance of either is still unclear; there is no consensus about the relevance of geographic patterns of expression (e.g., proximity of PD-L1 to immune-infiltrating lymphocytes or membranous versus cytoplasmic PD-L1) and PD-L1 evidence may also be affected by concurrent or prior treatment, including radiation or chemotherapy.12,13
Considering these data, reliable biomarkers to predict response and select patients to receive anti-PD-1 or anti-PD-L1 treatments are still lacking. It has been suggested that knowledge about PD-L1 expression needs to be applied in the context of T cell infiltrates being blocked by PD-1 receptor engagement.21 Tumeh et al.18 demonstrated that CD8 tumour-infiltrating lymphocytes (TIL) in the tumour microenvironment were associated with increased responsiveness to PD-1 inhibition. In this context, Teng et al.22 classified tumours as Type I (PD-L1-positive with TIL driving adaptive immune resistance), Type II (PD-L1- negative with no TIL indicating immune ignorance), Type III (PD-L1-positive with no TIL indicating intrinsic induction), or Type IV (PD-L1-negative with TIL indicating the role of other suppressors in promoting immune tolerance). Ock et al.23 comprehensively analysed the data on immunogenomic properties of tumours described in The Cancer Genome Atlas (TCGA) and classified the tumours based on PD-L1 status and TIL. The authors evidenced that high PD-L1 and CD8A expression (Type I tumour) was associated with a high mutational burden, PD-L1 amplification, and oncogenic viral infection. They also concluded that even when considering the cut-off of PD-L1 and TIL recruitment (assessed by CD8A) needed for clinical validation and further confirmation, this integrative analysis highlighted the importance of the assessment of both PD-L1 expression and TIL recruitment to predict responders to immune checkpoint inhibitors.23
OTHER PREDICTIVE FACTORS
NSCLC is associated with increased genomic instability and consequential mutations have the potential to generate tumour-specific antigens. Rizvi et al.24 evaluated the whole-exome sequencing of NSCLC patients treated with pembrolizumab. The authors showed a significantly improved efficacy of anti-PD-1 treatment for NSCLC with high non-synonymous mutation burden in terms of objective response rates (ORR) and durable clinical benefit (partial response or stable disease lasting ≥6 months). In one responder, neoantigen-specific CD8+ T cell responses matched tumour regression, suggesting that anti-PD-1 therapy could enhance neoantigen-specific T cell reactivity.24 This observation was consistent with the hypothesis that efficacy of anti-PD-1 therapy is largely related to recognition of neoantigens and it is expected to be higher in tumours with a high mutational load, particularly if >10 somatic mutations per megabase pair are present (corresponding to 150 nonsynonymous mutations within expressed genes).12,25
Smoking-induced lung cancers are also characterised by a higher number of mutations per megabase pair compared to tumours of never smokers. Govindan et al.26 described a median of 10.5 mutations per megabase pair (range: 4.9–17.6) in smokers and a median of 0.6 (range: 0.6–0.9) in never smokers. In this context, Rizvi et al.24 evidenced a greater benefit for tumours harbouring the molecular ‘smoking signature’, termed transversion-high (TH), compared to those with transversion-low (TL) tumours (ORR: TH 56% versus TL 17%; p=0.03; durable clinical benefit: TH 77% versus TL 22%; p=0.004; progression free survival [PFS]: TH median not reached [NR] versus TL 3.5 months; p=0.0001). In addition, KEYNOTE-001 trial investigators evidenced a response rate to pembrolizumab of 22.5% in current or former smokers compared to 10.3% in never smokers, further supporting that higher mutational burden associated with smoking contributes to an improved response to PD-1 inhibition.7 Finally, the hypothesis of a role for tumour mutation load and neoantigens in predicting the response to anti-PD-1 treatments is supported by recent evidence that tumours with mismatch-repair-deficiency achieve higher ORR and OS compared to mismatch-repair-proficient tumours.27
The presence of EGFR mutations and ALK rearrangements, which are usually associated with a lack of tobacco exposure, were associated with lower ORR to PD-1 inhibitors. Particularly, Gainor et al.28 evaluated 58 NSCLC patients treated with PD-1/PD-L1 inhibitors: objective responses were observed in 3.6% of EGFR-mutant or ALK-positive patients versus 23.3% of EGFR wild-type and ALK-negative or unknown patients (p=0.053). In addition, the ORR measured in never or light smokers (≤10 pack-years) was 4.2% versus 20.6% among heavy smokers (>10 pack-years; p=0.123). When studying advanced EGFR-mutant (n=68) and ALK-positive (n=27) patients, PD-L1 expression was observed in 24.0%, 16.0%, and 11.0% when cut-off values of ≥1.0%, ≥5.0%, and ≥50.0% tumour cell staining, respectively, were used; PD-L1 expression was also observed in 63.0%, 47.0%, and 26.0% of pre-tyrosine kinase inhibitor (TKI) biopsies using the same tumour call staining cut-offs, respectively.28 PD-L1 expression levels changed after resistance in 16 (28.0%) EGFR-mutant patients with paired, pre, and post-TKI-resistant biopsies (n=57), and concurrent PD-L1 expression (≥5.0%) and high levels of CD8+ TIL (Grade ≥2) were observed in 1 pretreatment (2.1%) and 5 resistant (11.6%) EGFR-mutant specimens; this finding was not noted in any ALK-positive, pre, or post-TKI specimens.28 Low rates of concurrent PD-L1 expression and CD8+ TIL within the tumour microenvironment can explain the low ORR to PD-1/PD-L1 inhibitors in NSCLC harbouring EGFR mutations or ALK rearrangements. Trials are ongoing to test if ORR to immunotherapy can be improved in these tumours if given in concurrence with TKI treatment.28
CURRENT IMMUNOTHERAPY APPROACHES AND FUTURE DIRECTIONS
Immunotherapy was approved for NSCLC treatment after impressive and durable responses, a low toxicity profile, and impact on OS as emerged from large randomised Phase III clinical trials.3,6,7 Immune checkpoints are proteins on lymphocyte surfaces and other immune cells, most notably on cytotoxic T cells that are able to induce stimulatory or inhibitory signals to trigger or reduce cellular adaptive immune responses when bound to their specific ligands.13 Many checkpoints have been described, including CTLA-4, PD-1 (and its ligand, PD-L1), B7-H3, B7x, T cell immunoglobulin (Ig) and mucin domain-containing molecule-3, and B and T cell lymphocyte attenuators.29 To date, the best characterised and most clinically studied are PD-1 and PD-L1 inhibitors, as shown in Table 1,21 as well as CTLA-4 checkpoints.13
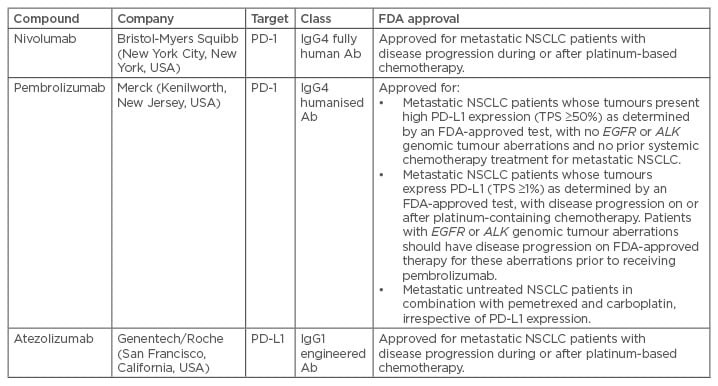
Table 1: Selected programmed death-1 or programmed death-ligand 1 inhibitors in advanced development of non-small cell lung cancer.
Ab: antibody; FDA: U.S. Food and Drug Administration; Ig: immunoglobulin; NSCLC: non-small cell lung cancer; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; TPS: tumour proportion score.
Adapted from Herzberg et al.21
PROGRAMMED DEATH-1 CHECKPOINT INHIBITORS
Nivolumab
Nivolumab is a fully human IgG4 immune checkpoint inhibitory antibody that binds to PD-1 and prevents its interaction with PD-L1 and PD-L2 (also called B7-DC or CD273). Following a Phase I pilot study by Brahmer et al.,30 nivolumab has been evaluated in Phase III CHECK-MATE-017 and CHECK-MATE-057 trials.2,3,30 CHECK-MATE-017 compared nivolumab to docetaxel in 272 advanced, pre-treated, squamous (SQ) NSCLC patients where PD-L1 positivity was not a requirement. This trial led to the first FDA approval of an immunotherapy for NSCLC, with a median OS of 9.2 months in the nivolumab arm (95% confidence interval [CI]: 7.3–13.3) versus 6.0 months in the docetaxel arm (95% CI: 5.1–7.3) and an ORR of 20% versus 9% (p=0.008). Median duration of response was NR with nivolumab (range: 2.9–20.5 months [an ongoing response at the time of analysis]) when compared with 8.4 months for docetaxel (range: 1.4 [with censored data because the patient received subsequent therapy] to 15.2 [an ongoing response at the time of analysis]).3
The CHECK-MATE-057 trial2 evaluated nivolumab versus docetaxel in 582 advanced, pretreated, non-SQ NSCLC patients. Median OS was, again, longer with nivolumab (12.2 months; 95% CI: 9.7–15.0) compared to docetaxel (9.4 months; 95% CI: 8.0–10.7). The ORR was also higher with nivolumab than docetaxel (19.0% versus 12.0%; p=0.02).2 PD-L1 was assessed retrospectively in prospectively collected pretreatment tumour biopsy specimens from the 582 patients who underwent randomisation; 455 (78%) had quantifiable PD-L1 expression. Cases of PD-L1-negative non-SQ NSCLC did not show a significant benefit with immunotherapy compared with the effect of chemotherapy that was seen in the SQ population (<1% PD-L1 OS HR: 0.90 [95% CI: 0.66–1.24]; <5% PD-L1 OS HR: 1.01 [95% CI: 0.77–1.34]; and <10% PD-L1 OS HR: 1.00 [95% CI: 0.76–1.31]).2
Pembrolizumab
Pembrolizumab is a humanised IgG4 monoclonal antibody targeting PD-1. The efficacy and safety of pembrolizumab was first assessed in NSCLC in the Phase I KEYNOTE-001 study by Garon et al.4 in 2015. Then, KEYNOTE-010,31 a randomised Phase III trial analogous to CHECKMATE-017 and 057, compared pembrolizumab at two doses, 2 mg/kg and 10 mg/kg every 3 weeks, to docetaxel in 1,034 patients. Only patients with ≥1% PD-L1-positive staining were enrolled, with 593 patients stratified by PD-L1 positivity using a 50% cut-off. Patients treated with pembrolizumab had a higher median OS than with docetaxel (10 mg/kg: 12.7 versus 8.5 months; HR: 0.61; p<0.0001; and 2 mg/kg: 10.4 months; HR 0.71; p=0.0008). When stratified by PD-L1 positivity, defined as a tumour proportion score ≥50%, survival benefits were more pronounced (HR: 0.50 and 0.54 for 10 mg/kg and 2 mg/kg cohorts, respectively), with a median survival of 17.3 and 14.9 months, respectively. In the subgroup analysis, both SQ and non-SQ histology favoured pembrolizumab, consistent with results from nivolumab clinical trials.31 Based on the unprecedented survival achieved in the PD-L1-positive population, the FDA approved pembrolizumab for second-line therapy in patients with a PD-L1 tumour proportion score ≥1%.
In KEYNOTE-021,32 front-line pembrolizumab was combined with carboplatin plus pemetrexed leading to an objective response in 55% of all patients; specifically, an objective response was observed in 54% of patients with ≥1% PD-L1 expression and in 80% of those with ≥50% PD-L1 expression. Updated results showed a significantly improved PFS with pembrolizumab plus chemotherapy versus chemotherapy alone (HR: 0.54; 95% CI: 0.33–0.88; p=0.0067), with a median (95% CI) PFS of 19 months (8.5–NR) versus 8.9 months (95% CI: 6.2–11.8). Median OS was NR (95% CI: 22.8–NR) for pembrolizumab plus chemotherapy and 20.9 months (14.9–NR) in the chemotherapy arm.32 These data led to accelerated approval, but continued authorisation for this indication may be contingent upon verification and description of clinical benefit in subsequent confirmatory trials.
Pembrolizumab was finally tested as a first-line therapy for metastatic NSCLC compared to different chemotherapy regimens in the Phase III KEYNOTE-024 trial.6 Patients (n=154) with ≥50% PD-L1-positive staining and no sensitising EGFR mutations or ALK rearrangements received pembrolizumab, while 151 patients received the investigator’s choice of platinum-based chemotherapy. PFS was the primary endpoint and was significantly longer in the pembrolizumab arm compared to chemotherapy (10.3 versus 6.0 months; HR: 0.50; p<0.001). OS was also significantly better in the pembrolizumab group (HR: 0.60; 95% CI: 0.41–0.89; p=0.005).6 Based on significant improvements in PFS and OS reported by this study, the FDA approved pembrolizumab as the first-line treatment of metastatic NSCLC patients whose tumours express PD-L1 on ≥50% of tumour cells.
Atezolizumab
Atezolizumab is a fully humanised engineered IgG1 monoclonal antibody against PD-L1. Based on OAK study results,7 in 2016 the FDA granted approval for its use in patients with advanced NSCLC whose disease progressed when treated with platinum-based chemotherapy. The OAK trial7 was a Phase III trial enrolling pre-treated SQ and non-SQ NSCLC patients who were randomly assigned to receive either atezolizumab (n=425) or docetaxel (n=425). Median OS was 13.8 months (95% CI: 11.8–15.7) in the atezolizumab arm compared to 9.6 months (95% CI: 8.6–11.2) in the docetaxel arm (HR: 0.73; 95% CI: 0.62–0.87; p=0.0003).7
CONCLUSION
Although checkpoint inhibitors have dramatically transformed the management of NSCLC, only a small percentage of patients currently benefit from PD-1 blockade therapy alone.16 Consequently, in order to increase ORR, novel treatment strategies are now under evaluation, including PD-1/PD-L1 inhibitors combined with other checkpoint inhibitors (e.g., CTLA-4, LAG-3, and T cell Ig and mucin domain-containing molecule-3), co-stimulatory checkpoints (e.g., OX40, GITR, and 4-1BB), immunomodulatory molecules (e.g., indoleamide 2,3-dioxygenase), chemotherapy, vaccines, and radiation.33
Hellmann et al.34 reported findings from the Phase I CHECK-MATE-012 study assessing the combination of nivolumab plus ipilimumab, an anti-CTLA-4 antibody, in advanced chemotherapy-naïve NSCLC; PD-L1 expression was not required for enrolment but it was analysed retrospectively. Results of two dose cohorts were reported: nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg either every 12 weeks or every 6 weeks. Confirmed ORR were described in 18 (47%; 95% CI: 31–64) of 38 patients in the ipilimumab every-12-weeks cohort and 15 (38%; 95% CI: 23–55) of 39 patients in the every-6-weeks cohort. Grade 3–4 treatment-related adverse effects (AE) occurred in 14 (37%) patients in the ipilimumab every-12-weeks cohort and 13 (33%) in the every-6-weeks cohort.
Treatment-related serious AE were reported in 12 (32%) patients in the ipilimumab every-12-weeks cohort and 11 (28%) patients in the every-6-weeks cohort. Treatment-related AE of any grade prompted treatment discontinuation in 4 (11%) patients in the every-12-weeks cohort and 5 (13%) in every-6-weeks cohort. Considering these data, larger studies are needed to establish the efficacy balanced with enhanced toxicity of these combinations.34
Finally, CHECKMATE-227,35 a randomised Phase III study of first-line nivolumab plus chemotherapy versus platinum-doublet chemotherapy, is currently underway. Collective results from these and similar studies will help give clarity regarding how different combination approaches will compare against checkpoint inhibitor monotherapy and platinum-based chemotherapy in distinct patient populations defined by PD-L1 expression alone. This could be even more important considering only about 30% of NSCLC patients have high (>50%) PD-L1 expression, and optimal first-line treatment still needs to be determined for the remaining 70% of patients with lower or absent PD-L1 expression.36
In conclusion, it is crucial to deepen the knowledge of immunological mechanisms and their biological impact. For both treatment optimisation and economic reasons, additional research is needed to identify relevant predictive biomarkers. Immunotherapy is undoubtedly a new option for NSCLC management but results from all ongoing clinical trials will further clarify its best application in terms of combinations or sequences of treatments.








