Abstract
Introduction: Gene expression assays, such as the MammaPrint® (Agendia, Amsterdam, the Netherlands) 70-gene signature, are increasingly used by oncologists to understand breast cancer biology and improve treatment planning. This study assesses the utility of MammaPrint genomic risk in predicting treatment outcomes for women with breast cancer in a retrospective German cohort with a 10-year follow-up, treated based on clinicopathological features alone.
Methods: The sample set of 117 tumours from the ‘Patients Tumour Bank of Hope’ (PATH) biobank with 10-year follow-up were classified using MammaPrint into high or low risk of distant metastasis. Patients were previously treated according to St. Gallen and Adjuvant! Online high- or low-risk criteria. Statistical analyses compared overall survival (OS) and treatment outcomes between clinical and genomic risk groups.
Results: Among the 78 patients with clinically high-risk tumours, 50% (39) were reclassified as MammaPrint low risk. In total, 57.3% (67/117) patients with MammaPrint low-risk tumours demonstrated a significantly higher 10-year OS of 93.4%, irrespective of nodal status, compared to patients with MammaPrint high-risk tumours (71.2%; p=0.001). Chemotherapy improved OS in patients with MammaPrint high-risk tumours by 29.4%, but not for patients with MammaPrint low-risk tumours (p=0.016). Discussion: The findings confirm the prognostic utility of MammaPrint for identifying genomically low-risk patients who may safely omit chemotherapy while suggesting genomically high-risk cases may benefit from chemotherapy. By providing a more precise assessment of cancer risk than traditional clinicopathological methods alone, MammaPrint may help reduce unnecessary treatments and improve long-term quality of life for patients diagnosed with early-stage breast cancer.
Key Points
1. The prognosis in early breast cancer is based on the underlying personal risk of recurrence.2. To receive more detailed information on the breast cancer biology of a given patient, more information is needed for individualised treatment recommendations to avoid unnecessary over- and undertreatment.
3. Multi-gene assays such as the MammaPrint 70-gene signature provide this information to allow classical prognostic information such as ‘clinical risk’ into ‘genomic risk’ to be transformed to gain more information on tumour biology and optimise the early breast cancer therapy.
INTRODUCTION
In modern oncology, the utilisation of gene expression assays has driven the development of personalised treatment strategies for patients with breast cancer, offering a deeper understanding of the molecular underpinnings of the disease. The most frequently used genomic breast cancer tests in clinical practice include MammaPrint® (Agendia, Amsterdam, the Netherlands), Oncotype DX® (Exact Sciences, Madison, Wisconsin, USA), Prosigna™ (NanoString Technologies, Inc., Seattle, Washington, USA), and EndoPredict (Eurobio Scientific, Essonnes, France).1 Among these, only MammaPrint and Oncotype DX are validated by prospective randomised Phase III trials for hormone receptor positive (HR+) and HER2-negative (HER2-) disease.2,3,4
Prospective non-inferiority trials designed to validate these tests include TAILORx and RxPONDER, which assess the de-escalation of chemotherapy using the 21-gene Oncotype DX assay in breast cancer treatment. The TAILORx trial showed that, at 9-year follow-up, women with Oncotype intermediate scores often do not benefit from additional chemotherapy when they have HR+, HER2-, and lymph node-negative (LN-) breast cancer.4 The RxPONDER trial’s 5-year follow-up data extended the assay’s evaluation to node-positive (LN+) patients, suggesting that some might avoid chemotherapy based on their gene expression profiles.2
MammaPrint, a 70-gene recurrence risk signature assay, evaluates the expression of cancer-related genes to classify early-stage breast cancer tumours into high-risk or low-risk categories for developing distant recurrence. This prognostic tool was specifically designed for patients with both HR+ and HR-, HER2- breast cancer with either LN- or LN+ disease (up to three positive nodes).5-9
The prospective, randomised, non-inferiority trial designed to validate MammaPrint, MINDACT (NCT00433589),10 identified patients with a low genomic risk of breast cancer recurrence who might safely omit chemotherapy from their treatment plan. With a median follow-up of 8.7 years, the trial demonstrated the capability of MammaPrint to identify clinically high-risk patients who exhibit low genomic risk and can achieve excellent outcomes with endocrine therapy alone, without the need for chemotherapy. The study found comparable distant metastasis-free survival rates between patients who received endocrine therapy alone and those who underwent chemotherapy, with a negligible difference of 0.9% at the 5-year mark, regardless of whether the cancer had spread to lymph nodes.3,6
Complementing the findings of the MINDACT trial, a German retrospective multicentre pilot study using samples from the ‘Patients Tumour Bank of Hope’ (PATH) further explored the concordance between treatment recommendations based on MammaPrint and those based on clinical risk classifications at the time of diagnosis. The study found that MammaPrint reclassified 40% of the patients in the PATH study population, resulting in different treatment recommendations for these patients compared to the initial plan.11
Building on these initial results, the authors analysed the impact of MammaPrint genomic classification compared to clinical risk assessment on treatment planning and 10-year outcomes in patients from the PATH cohort. The rationale of this analysis was to evaluate the predictive utility of MammaPrint for long-term treatment planning and therapeutic efficacy of adjuvant chemotherapy and/or endocrine therapy 10 years after presentation for early-stage breast cancer.
MATERIALS AND METHODS
Patient Population
Tumour samples of 140 German patients who had been diagnosed with Stage I and II early-stage breast cancer between November 2005–April 2008 were obtained from the PATH biobank. Biopsies of the primary tumour were snap-frozen at the clinical site and stored at the PATH Tumour Bank of Hope under specific conditions. All patients had undergone standard surgical procedures, including modified radical mastectomy or breast-conserving surgery including axillary clearance and radiotherapy according to national guidelines. Initially, the necessity for adjuvant therapy was determined based on clinical risk factors, including factors such as tumour size, nodal status, histological grade, and hormone receptor status.
Clinical Risk Classification
Histopathological data, including tumour grading, were assessed according to the Elston and Ellis method.12 Oestrogen receptor (ER) and progesterone receptor (PR) status were determined through immunohistochemistry, with tumours classified as ER/PR-positive if more than 10% of the cell nuclei stained positive for these receptors. The prognostic value of MammaPrint was assessed in comparison with the St. Gallen criteria, which were defined at the 9th St. Gallen Consensus Meeting in 2005. These criteria take several clinicopathologic factors into account, including the size of the primary tumour, patient age, histological grade, hormone receptor status, peritumoiral vascular invasion, and HER2 status. A significant number of the patient cohort was in the intermediate-risk category according to the St. Gallen criteria. The clinical risk of developing distant metastases was further assessed using Adjuvant! Online (site inactive since 2015) and included patient age,13 ER status, nodal status, tumour size, and histological grade. Adjuvant! Online provided estimates of overall survival (OS), breast cancer-specific survival, and event-free survival.
MammaPrint Laboratory Assay
As described in the original publication RNA extraction from the tissue provided by the PATH study bank was conducted at Agendia, with the process blinded to clinical-pathologic data. Custom-designed arrays (Agilent Technologies, Santa Clara, California, USA) were utilised to generate microarray gene expression data according to established protocols.14 The 70-gene MammaPrint signature identified genomically low-risk tumours by the previously established threshold, defined as the patient’s likelihood of 5-year distant metastasis-free survival surpassing 90%.8 All other tumours were classified as high-risk.
Statistical Analysis
The primary objective was to analyse patient outcomes 10 years after an early-stage breast cancer diagnosis, focusing on the prognostic impact of MammaPrint. The impact of MammaPrint risk, lymph node status, and adjuvant chemotherapy treatments were considered. Differences in clinical characteristics were analysed by Chi-Squared or Fisher’s Exact tests. Differences in OS and death by any cause were assessed using Kaplan-Meier survival analysis. Log-rank tests were conducted to determine significant differences in 10-year survival rates between groups, denoting p-values of 0.05 or less as statistically significant.
RESULTS
Patient Population
The study population consisted of 117 patients with 10-year follow-up and a median age of 62.5 years (Table 1). The majority of patients were over 55 years old, accounting for 64.1% of the total cohort.
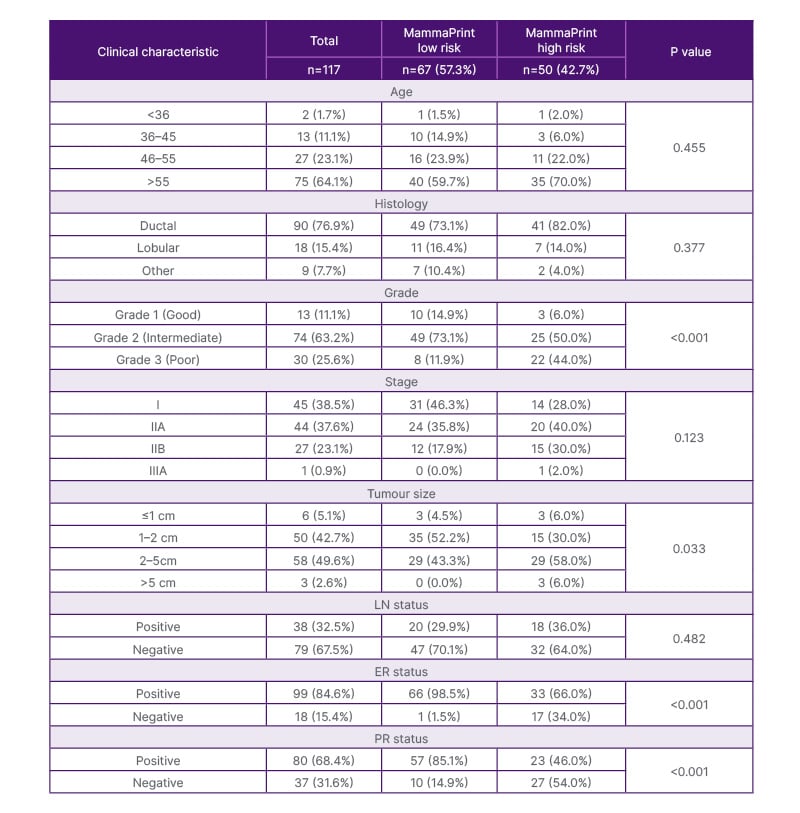
Table 1: Clinical characteristics.
Data represented as n (%), unless otherwise specified. Differences in groups were assessed by using Pearson’s Chi-squared tests or Fisher’s exact test. Statistical significance was defined as P<0.05.
ER: oestrogen receptor; LN: lymph node; n: number of participants; PR: progesterone receptor.
The median tumour size was 15.0 mm, and nuclear grading was most frequently Grade 2 (63.2%). In addition, 67.5% of patients presented with negative nodes (LN-) and 76.9% of tumours were classified as invasive ductal cancers. The MammaPrint testing revealed that 57.3% (67) patients had tumours classified as genomically low risk, while 42.7% (50) patients were classified as having MammaPrint high-risk tumours. Age, tumour stage, and nodal status were similar between both genomic risk categories. In contrast, patients with MammaPrint high-risk tumours exhibited a significantly higher frequency of Grade 3 tumours (44.0%) compared to those with MammaPrint low-risk tumours (11.9%; p<0.001). Among PATH participants with 10-year outcomes data, 42.7% (50/117) of tumours were genomically discordant with clinical risk, with 50.0% of clinically high-risk tumours reclassified as MammaPrint low risk and 28.0% of clinically low-risk tumours reclassified as MammaPrint high risk (Supplementary Figure 1).
Systemic Therapy
Adjuvant systemic therapy was indicated in 95 of the 117 patients, consisting of either chemotherapy (10/95; 10.5%), endocrine therapy (42/95; 44.2%), or both (43/95; 45.3%). One patient did not receive any adjuvant systemic therapy, and in 21 cases, treatment was unknown. Out of 67 MammaPrint low-risk patients, 43.3% (29) were treated with chemoendorince therapy (chemotherapy plus endocrine therapy), and 43.3% (29) only received endocrine therapy. Within the MammaPrint high-risk group, 13 out of 50 patients (26.0%) only received endocrine therapy, 10 (20.0%) received chemotherapy only, and 14 (28.0%) received chemotherapy plus endocrine therapy. In a cohort treated based on clinical risk assessments only, 44.8% (43/96) of patients with recorded treatments received a therapy not in line with MammaPrint recommended treatments (Table 2).
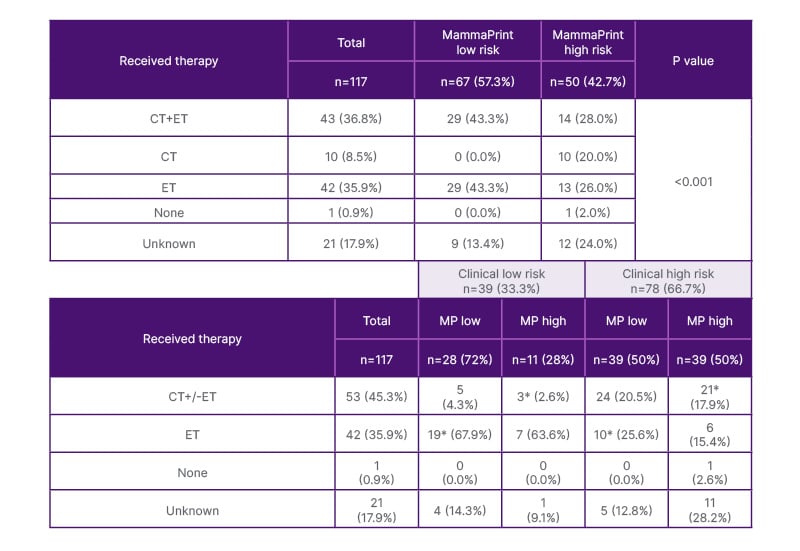
Table 2: Adjuvant treatments for clinical and genomic risk groups.
Data represented as n (%), unless otherwise specified. Differences in groups were assessed by using Pearson’s Chi-squared tests. Statistical significance was defined as P<0.05.
*Indicates recommended treatments based on MammaPrint result. Patients received treatments based on clinical risk factors, according to German national guidelines and standard of care treatments at the time of diagnosis.
CT: chemotherapy; ET: endocrine therapy; MP: MammaPrint; n: number of participants.
10-Year Survival
The median follow-up period for OS was 10 years. At 10 years of follow-up, there were a total of 17 recorded death events. Among these, four deaths occurred in patients with MammaPrint low-risk tumours (6.0%; 4/67), while 13 deaths were observed in patients with MammaPrint high-risk tumours (26.0%; 13/50). Patients with MammaPrint low-risk tumours displayed a remarkable 10-year OS rate of 93.4% (95% CI: 87.1–99.7), which was significantly higher when compared to those with MammaPrint high-risk tumours (71.2%; 95% CI: 57.9–84.5; p=0.001) (Figure 1A). Among patients with MammaPrint low-risk tumours, 10-year OS was the same regardless of nodal status (93.3%; low-risk LN-: 95% CI: 85.9–100; and node-positive: 95% CI: 80.8–100) (Figure 1B). In contrast, the MammaPrint high-risk LN+ group exhibited a significantly lower 10-year OS rate of 40.4% (95% CI: 16.3–64.5) compared to the high-risk LN- group, with a rate of 89.0% (95% CI: 77.2–100), and to MammaPrint low-risk groups (p<0.001).
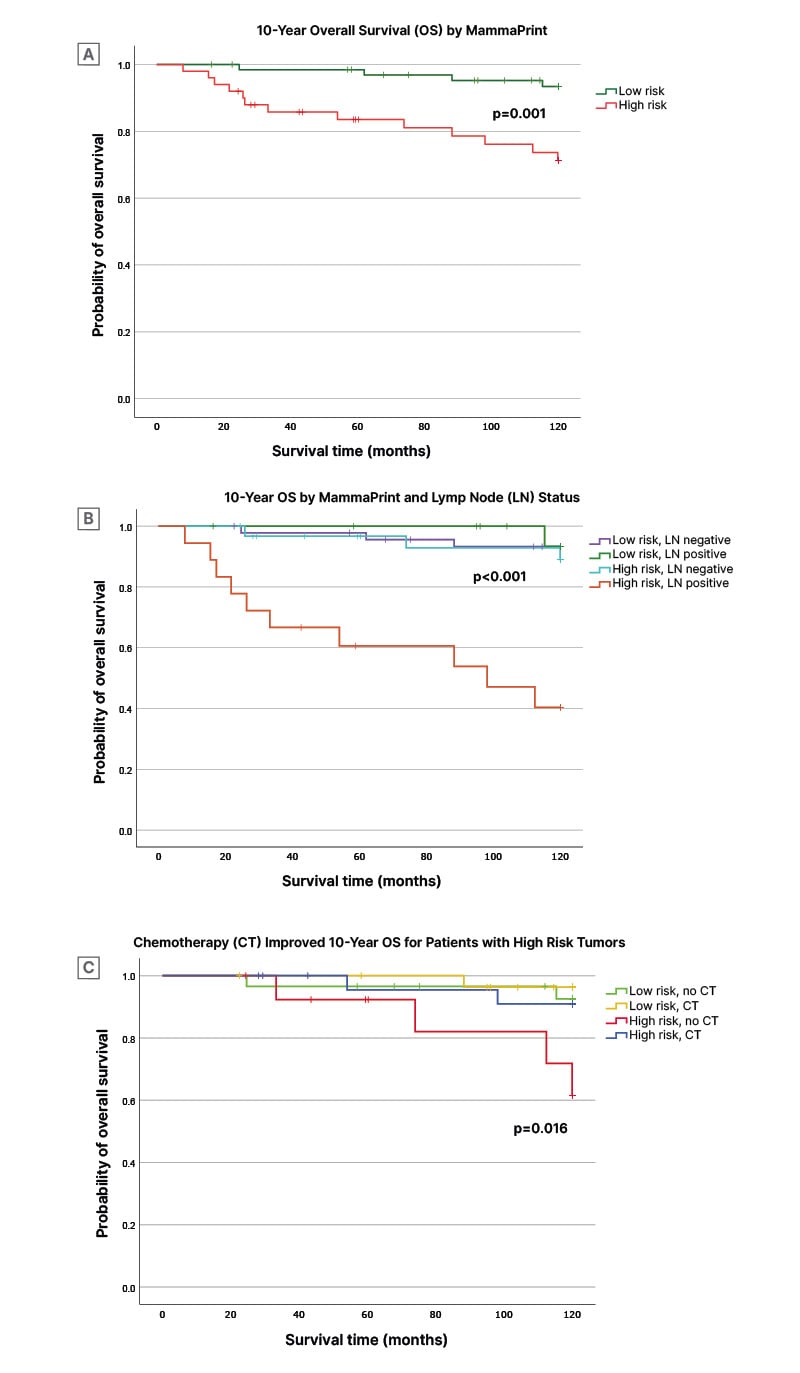
Figure 1: 10-year overall survival stratified by mammaprint risk, lymph node status, and chemotherapy treatment.
A) 10-year OS stratified MammaPrint low (green) and high (red) risk of distant metastasis.
B) 10-year OS stratified MP low risk/LN- (purple), MP low risk/LN+ (green), MP high risk/LN- (light blue), and MP high risk/LN+ (orange).
C) 10-year OS stratified MP low risk no CT (green), MP low risk with CT (yellow), MP high risk no CT (red), and MP high risk with CT (blue grey). Significance between groups was assessed by a log-rank test. Statistical significance was defined as p<0.05.
OS: overall survival; MP: MammaPrint; LN+/-: lymph node positive/negative; CT: chemotherapy.
Among patients with MammaPrint low-risk tumours, OS was similar over time between those who received endocrine treatment only and those who received chemotherapy with or without endocrine therapy. At 10 years, OS rates among the low-risk group were 92.5% (95% CI: 82.5–100) for endocrine-only treated and 96.3% (95% CI: 89.2–100; p=0.572) for chemotherapy-treated patients (Figure 1C). In contrast, patients with MammaPrint high-risk tumours who did not receive any chemotherapy had significantly poorer survival at 10 years with an OS rate of 61.5% (95% CI: 31.5–91.5) compared to patients with MammaPrint high-risk tumours that received chemotherapy (10-year OS rate: 90.9%; 95% CI: 78.9–100; p=0.016) and to MammaPrint low-risk groups.
DISCUSSION
This study evaluated the prognostic utility of MammaPrint genomic classification at 10 years of follow-up in patients with early-stage breast cancer tumour samples donated from the German PATH cohort (N=117). These findings reinforce existing studies demonstrating the utility of MammaPrint for treatment planning and prognosis. The observed 10-year OS for all patients with MammaPrint low-risk tumours was more than 93%, regardless of lymph node involvement. This aligns with the MINDACT trial, which reported excellent 8-year OS of >94% for MammaPrint low-risk groups irrespective of treatment and lymph node status.3 Here, the authors observed no significant differences in 10-year OS of patients with MammaPrint low-risk tumours regardless of the use of chemotherapy. Knauer et al.15 also showed favourable outcomes of 97% breast cancer-specific survival (BCSS) 5 years after diagnosis for the MammaPrint low-risk group treated with endocrine therapy alone.15 Additionally, the authors found that patients with MammaPrint low-risk tumours had the same 10-year outcomes with either LN+ or LN- disease (93.3% OS). The MINDACT trial, which included patients with LN+ disease, found that women with clinically high/MammaPrint low-risk tumours (approximately 47% LN+ samples) demonstrated similar 8-year OS of 94.7%, compared to 96.5% for women with clinically low/MammaPrint low-risk tumours (approximately 6% LN+ samples).3 These data are in alignment with existing research suggesting that the omission of chemotherapy and treatment with endocrine therapy alone are sufficient to sustain excellent survival outcomes for patients with MammaPrint low-risk tumours, even in cases of LN+ or clinically high-risk status.
With strong evidence of high survival probability for MammaPrint low-risk patients, increased utilisation of MammaPrint for treatment planning can help spare patients with genomically low-risk tumours from adverse effects of chemotoxicity and improve long-term quality-of-life for survivors. Previously, other studies have observed large proportions (30–50%) of patients with clinically high-risk features identified as low risk by MammaPrint;3,16 and therefore genomically eligible for chemotherapy omission. In the PATH cohort, it was originally observed that approximately 40% of patients had discordant clinical and MammaPrint Risk results.11 In this 10-year follow-up population, MammaPrint identified 50% (39/78) of clinically high-risk tumours as genomically low risk. Among this subset, most patients (24/39) were treated with chemotherapy but could have had equally favourable outcomes, lower cytotoxicity, and reduced treatment costs with endocrine therapy alone.17
In contrast to MammaPrint low-risk 10-year outcomes, the MammaPrint high-risk group had significantly lower OS (71.2%; p=0.001). These data are consistent with worse 10-year BCSS outcomes observed in MammaPrint high-risk (<82%) compared to low-risk (>92%) in the STO-3 trial for patients with node-negative disease.18 This study also observed the poorest survival for MammaPrint high-risk LN+ tumours. Similarly, Mook et al.19 observed a 20% decrease in 10-year BCSS for patients with MammaPrint high-risk compared to MammaPrint low-risk tumours with 1–3 positive lymph nodes.19 Additionally, the MINDACT trial observed a worse 8-year OS of 90.1% for women with clinically high/MammaPrint high-risk tumours (approximately 26% LN+ samples) compared to 94.7% for clinically high/MammaPrint low-risk tumours (approximately 47% LN+ samples). These results suggest that MammaPrint risk analysis provides a more accurate prognosis for LN+ disease compared to relying on clinical risk alone. These data highlight the importance of treating patients with MammaPrint high-risk tumours and LN+ with chemotherapy, while patients with MammaPrint low-risk tumours and LN+ may be safely spared from the toxicities associated with chemotherapy.
Moreover, the authors observed poorer 10-year survival in patients with MammaPrint high-risk tumours who did not receive chemotherapy, with a notable 29% drop in survival compared to patients with MammaPrint high-risk tumours who received chemotherapy. A meaningful chemotherapy benefit was also reported by Knauer et al.,15 in which adjuvant chemotherapy treatment led to significantly better 5-year distant disease-free survival of 88% for patients with MammaPrint high-risk tumours who received chemotherapy compared to 76% for those not treated with chemotherapy.15 In support of these findings, neoadjuvant studies have also demonstrated an increase in chemosensitivity in MammaPrint high-risk tumours. In the NBRST trial, greater chemosensitivity in the form of pathological complete response (pCR) was observed for patients with MammaPrint high-risk tumours.20 Of the patients who achieved pCR with neoadjuvant chemotherapy, 97% had tumours characterised as MammaPrint high risk. Further investigation into the mechanisms of chemosensitivity in MammaPrint high-risk tumours is warranted.21
While this retrospective observational analysis is non-randomised and limited by the small sample size of 117 patients receiving standard-of-care treatments based on clinical-pathological features, the 10-year outcomes provide valuable long-term survival data that reflect existing study results for patients with early-stage breast cancer with HR+HER2- tumours.
Furthermore, since its inception, clinical studies utilising MammaPrint have identified cohorts of women with genomically low-risk disease with indolent disease. 18,22,23 Unlike Oncotype DX, MammaPrint’s ultra low-risk category offers a more distinct and clinically relevant stratification of patients that show excellent survival outcomes without extended endocrine therapy, underscoring the potential over-treatment of these patients, not only with chemotherapy but also with extended endocrine therapy.
Here, the authors demonstrated the utility of MammaPrint for treatment planning based on 10-year outcomes and reinforced previous evidence advocating for chemotherapy benefits when treating MammaPrint high-risk tumours. The data further strengthens the rationale for omitting chemotherapy in the management of MammaPrint low-risk tumours, aiming to reduce the detrimental effects of chemotoxicity while maintaining favourable long-term survival outcomes.







