Abstract
With the recent growth in oral anticancer agents (OAA), pharmacists working in the community have recognised the urgent need to develop safe and effective systems to administer and manage these drugs. For community pharmacists, education regarding OAA can be challenging, with a number of international surveys showing that many believe they have received inadequate education regarding OAA and feel uncomfortable educating their patients about these drugs. Patients prescribed OAA have also reported feeling unsupported, and this lack of support could lead to both under and overadherence to OAA, with an impact on efficacy and adverse events. Poor adherence can result in disease progression, treatment complications, reduced functional ability, and premature death.
The current review, written by international authors from Europe, North America, and East Asia, set out to identify worldwide initiatives to support community pharmacists working with patients taking OAA. The authors identified one project, the Oral Anticancer Therapy – Safe and Effective initiative, that was developed in Germany in 2011 to aid community pharmacists in their interactions with patients prescribed OAA. The initiative, which has been rolled out across Germany, includes the creation of training programme content that can be delivered at regional meetings and monographs, which can be downloaded to educate both community pharmacists and their patients about individual OAA. As part of the Empowering Patients to Improve Health Care for Oral Chemotherapy (EPIC) programme, the European Society of Oncology Pharmacy (ESOP) has extended the German initiative to Slovenia and Estonia, with plans to launch the scheme in additional European countries in the autumn of 2018. Ultimately, it is hoped that better support of cancer patients in the community will improve adherence to OAA.
GROWTH IN ORAL ANTICANCER AGENTS
In recent years, oncology therapies have undergone a paradigm shift from being delivered mainly as intravenous (IV) chemotherapy in hospitals and outpatient clinics to oral anticancer agents (OAA) taken at home. This change has had wide-ranging repercussions for the workload and care delivery model of pharmacists, in particular those working in the community.
In 1995, only six OAA were available, but by 2007 >12 were in use and between 2015 and 2017 23 were approved by the U.S. Food and Drug Administration (FDA).1,2 A similar picture holds for Europe, with two OAA being approved by the European Medicines Agency (EMA) between 2001 and 2003 compared to 16 OAA between 2015 and 2017 (Figure 1). The way OAA have revolutionised oncology can be appreciated by considering that 25 OAA were approved by the FDA between 2011 and 2014, compared with 18 cancer IV agents during the same period.3 It has been shown that the total drug expenditure in the USA on targeted therapies (almost exclusively OAA) increased from 26% in 2010 to 40% in 2016,4 and it is estimated that 25–30% of all haematological oncology drugs currently in development are orally administered, small molecules.5 OAA, which can be delivered as tablets, capsules, or a liquid, range from traditional endocrine and cytotoxic therapies to biological therapies targeted at cell surface proteins or mechanisms specific to cancer biologic pathways.
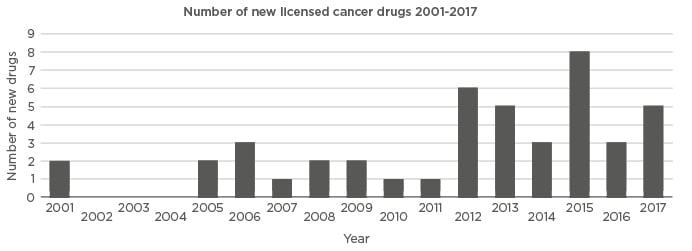
Figure 1: Graph showing the year that different oral anticancer agents were licensed in Europe. Courtesy of Klaus Meier.
2001: capecitabin, imatinib; 2005: anagrelide, erlotinib; 2006: dasatinib, sorafenib, sunitinib; 2007: lenalidomide; 2008: lapatinib, nilotinib; 2009: gefitinib, thalidomide; 2010: pazopanib; 2011: abiraterone; 2012: axitinib, crizotinib, ruxolitinib, tegafur-kombi, vandetanib, vemurafenib; 2013: afatinib, dabrafenib, enzalutamide, regorafenib, vismodegib; 2014: cabozantinib, ibrutinib, idelalisib; 2015: carfilzomib, ceritinib, cobimetinib, lenvatinib, nintedanib, olaparib, panobinostat, trametinib; 2016: osimertinib, palbociclib, tipiracil; 2017: alectinib, ixazomib, ribociclib, tivozanib, venetoclax.
As a direct result of the growth in the number of OAA available, cancer patient management has evolved from a process that was controlled and monitored by clinicians and nurses in hospitals and outpatient clinics, to one that involves patients and their caregivers having the majority of treatment responsibility.6 For patients, the convenience of oral therapy offers the potential to improve quality of life; with OAA, patients need fewer hospital and outpatient appointments; thus, they are required to spend less time away from their work and families. An additional advantage is that there is no requirement for IV access, which can cause complications such as extravasations, venous sclerosis, infections, and injection site reactions.7 Many studies have suggested that patients prefer OAA to IV therapy;8-11 however, although patients can feel empowered by taking direct responsibility for managing their treatment, this responsibility can prove overwhelming for sick patients who lack reliable support from families and friends.12 Candidates for OAA need to be well-motivated, have good health literacy, and be able to manage complex regimens. There is also a requirement for a healthy food intake and gut function, with minimal nausea and vomiting, since the bioavailability of oral agents is greatly affected by diet.13
The introduction of OAA to the community also offers advantages to health services, including the potential for cost savings as a result of reduced hospital admissions or outpatient infusions. Indeed, a UK time and motion study showed that switching from IV chemotherapy to OAA allowed a seven-fold increase in the number of patients treated.14 In addition, surveys have shown that both patients and healthcare professionals perceive OAA to be safer than IV cancer therapy.15,16
However, patients often do not fully appreciate that OAA can have life-threatening levels of toxicity and often incorrectly believe that they are similar to vitamins or antibiotics. OAA treatment can result in fatalities; for example, in the UK, a National Patient Safety Agency (NPSA) alert on oral anticancer medicines was issued in 2008 following three deaths and 434 safety incidents that occurred between November 2003 and July 2007.17 Many stakeholders do not fully appreciate that in addition to the hazards posed to patients, there are risks for family caregivers and healthcare personnel involved in handling OAA. Studies have found that up to two-thirds of staff handling these medications showed measurable amounts of the agent in their urine.18,19
In the hospital and clinical settings, safety systems for IV chemotherapy have been well-developed, with prescribers using electronic order sets, pharmacists verifying and preparing treatments, and nurses educating patients and delivering therapy. This infrastructure creates the ability to assess toxicity and adjust therapy at the point of delivery.20 In contrast, when patients are prescribed OAA in the community, safety and support systems may not be in place. Prescriptions may be completed by community pharmacists who often have not been provided with adequate information about the patient’s disease, weight, height, test results, concurrent medications, or dietary habits. One concern is that when cancer therapies are taken orally there may be a lack of independent checking, with safeguards routinely adopted for IV chemotherapy not used for oral chemotherapy.
A study from the USA reported the results of a survey of OAA safety practices at 42 USA cancer centres and found that information required on a prescription, such as diagnosis, cycle number, prescriptions checked by other clinicians, calculation of body surface area, or dose per m2 per body surface area, was variable.21 The study showed that 10 centres had no formal process for monitoring adherence and 10 centres reported at least one serious adverse event in the previous year.21 Additionally, a Canadian study showed that a total of 57 systematic checks were identified for IV chemotherapy but only six for oral chemotherapy.22
When patients take medications at home there are risks of poor adherence. One study involving 119 patients taking OAA for various types of cancer showed that of the 33 patients who were nonadherent to OAA, 20 were overadherent and 13 underadherent.23 Without comprehensive toxicity management and adherence programmes, oncologists may be assessing responses to therapy without knowing the degree of treatment adherence. If clinicians are unaware that OAA are not being used as prescribed, disease progression may be inappropriately attributed to lack of efficacy and clinicians may change treatments unnecessarily.24
Undoubtedly, there is an urgent need to develop effective systems to improve the quality and safety of OAA delivery and management. Pharmacists, in particular those working in the community, face challenges to update their knowledge, reduce medication errors, provide safe handling of drugs, manage side effects, and deliver education to patients and their caregivers. Internationally, there is a need to develop infrastructures to ensure that patients receiving OAA in the community receive standards of care that are equivalent to those receiving IV therapy in hospitals.
INCREASED ROLE FOR COMMUNITY PHARMACISTS
The healthcare settings in which OAA are dispensed vary widely around the world. In the UK, Belgium, and Poland, all OAA are dispensed by hospital pharmacy departments, while in other European countries, including Germany, France, Spain, Ireland, Slovenia, Croatia, and Estonia, community pharmacists play an important role in OAA delivery (Figure 2). In the USA, the dispensing of OAA is largely restricted to specialist pharmacies that employ pharmacists with disease-specific expertise in oncology,25 and, in Canada, a hybrid system exists in which cancer agencies dispense in the western provinces and community pharmacies dispense in the eastern provinces.26 In Japan, both hospital and community pharmacists provide OAA to patients.27
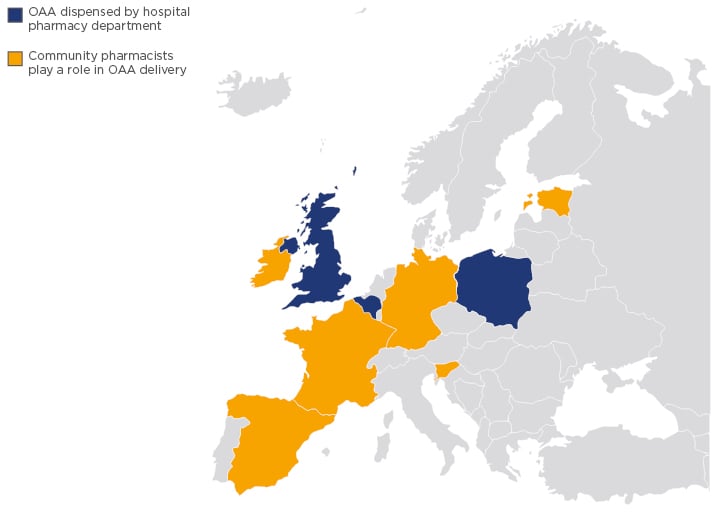
Figure 2: Map of Europe showing the countries in which oral anticancer agents are dispensed by hospital pharmacy departments and in which community pharmacists play an important role.
OAA: oral anticancer agents.
Educational systems regarding OAA are starting to be put in place for hospital pharmacists. For example, in the UK, hospital pharmacists prescribing OAA are required to have received accreditation from the British Oncology Pharmacy Association (BOPA), and in the USA the Board of Pharmacy Specialties (BPS) provides board certification for oncology pharmacists. In Japan, the completion of an outpatient chemotherapy pharmacy certification programme, known as the accredited pharmacists of ambulatory cancer chemotherapy (APACC) and offered to both hospital and community pharmacists, is one of the requirements for receiving OAA healthcare reimbursement fees.27
Standards for OAA have also been produced by a range of organisations, including The American Society of Clinical Oncology (ASCO) and the Oncology Nursing Society (ONS),28 the Japanese Society of Pharmaceutical Oncology (JASPO), and the Canadian Association of Provincial Cancer Agencies (CAPCA).29 These outline a set of expectations and frameworks for individual healthcare providers in relation to oral chemotherapy, and JASPO has published guidance on oral therapy collaborations between hospital and community pharmacists.30 However, universal guidelines for managing adherence to oral chemotherapy have yet to be established.
Most undergraduate pharmacy curriculums only touch on OAA briefly, with the result that most community pharmacists have gaps in their knowledge. With OAA becoming more widely used, community pharmacists, even in European countries where the prescription and control of OAA is controlled by hospital teams, should be educated in the field to participate in the compliance and control of the side effects of the drugs.
Surveys undertaken in a range of different countries revealed that community pharmacists felt concerned by their lack of oncology training and did not feel equipped to ensure the safe use of OAA.
A survey of 283 Japanese community pharmacists, which took place between May and June 2014, found that only 6–10% felt they had received adequate education and training regarding OAA. Furthermore, although 81% of respondents had attended at least one continuing education event relating to oncology in the past 2 years, only 54% felt comfortable dispensing OAA and 40% felt comfortable educating patients about OAA. Only two pharmacies in the survey (0.3%) had a certification related to oncology pharmacy.27
A similar theme emerged from Canada, with a survey of 352 community pharmacists finding that 13.6% of respondents felt they had received adequate oncology education at undergraduate level, 19.0% had attended a continuing education event relating to oncology in the past 2 years, 24.0% were familiar with the common doses of OAA, and only 9.0% felt comfortable educating patients.31 Similarly, in Ireland, two-thirds of community pharmacists surveyed felt they did not have sufficient information available to safely dispense prescriptions for OAA and three-quarters felt the current Irish system placed patients at risk.32
Patients have also reported feeling unsupported; a survey by the German Society for Oncology Pharmacy (Deutsche Gesellschaft für Onkologische Pharmazie [DGOP]) of 427 patients receiving OAA from community pharmacists found that one-third of respondents had not received any advice, although around one-half would have welcomed it.33 Taken together, such surveys demonstrate the need to develop education programmes to support community pharmacists in playing a greater role in the care of patients receiving OAA.
IMPORTANCE OF PATIENT ADHERENCE
One of the greatest concerns related to dispensing OAA in the community is that the lack of supervision will affect patient adherence, resulting in both under and overadherence, and that this will in turn reduce efficacy and increase the risk of adverse events. Adherence implies a collaborative approach to decision-making between patients and healthcare staff, while compliance has the connotation of a passive role for patients in receiving and following medical advice. Optimal adherence is achieved “if no doses are missed, no extra doses are taken, and no doses are taken in the wrong quantity or at the wrong time”.34
Studies across a range of different disease areas have showed that approximately half of all patients do not take their medications as prescribed.35 A systematic review undertaken between January 2003 and June 2015 showed that rates of adherence for OAA varied from 46–100% depending on patient sample, medication type, follow-up period, assessment measure, and calculation of adherence.36 Adherence to OAA appears higher than for other medications, most likely due to cancer being perceived by patients as a life-threatening disease. The significance of nonadherence varies markedly between OAA. Taking the example of breast cancer hormone therapies, patients will be far less compromised by missed doses of tamoxifen (half-life: 7–14 days)37 than missed doses of the aromatase inhibitors letrozole and anastrozole (half-life: 2 days and 27 hours, respectively).38 Poor adherence to OAA can result in unnecessary disease progression, treatment complications, reduced functional abilities, lower quality of life, and premature death.39 For example, an imatinib study in chronic myeloid leukaemia showed that 23% of patients with suboptimal responses were nonadherent compared to 7% nonadherence with optimal responses.40 Other studies demonstrated that tamoxifen patients who completed <70% of their prescriptions had an increased risk of death41 and that renal cell carcinoma patients who did not adhere to axitinib and everolimus showed significant decreases in progression-free survival.42
Reasons for nonadherence are attributed to three main themes: personal patient factors (belief in the treatment and emotional state), treatment factors (side effects, complexity of the treatments, and costs), and healthcare provider factors (relationship with the healthcare professionals and prescribing practices).34 Focussing on treatment factors, frequency, severity, and types of side effects of medications are all likely to affect adherence. This can be especially the case for OAA, which often have novel modes of action with rare and unexpected side effects;43 for example, OAA can cause life-threatening side effects, including neutropenic sepsis and diarrhoea, and other side effects such as hypertensive episodes and extreme skin toxicities. Adverse drug reactions to OAA are common; a study reviewing 1,061 prescriptions for OAA at an academic outpatient cancer centre over 1 year reported that, within 90 days of initiation, 80% of patients had experienced treatment-related toxicities secondary to an OAA, with 36% classified as severe and 17% requiring hospitalisation.43
Comorbidities also complicate treatment. A study showed oncology patients have an average of 3.2 comorbid conditions for which they take 10–12 medications,44 all of which having the potential to interact with OAA. An academic outpatient centre study found that, in addition to OAA, patients were prescribed a mean of 10.9 medications and had a mean of 2.1 major drug interactions.43 Taking the example of erlotinib, drugs that increase the pH of the upper gastrointestinal tract may alter its solubility and reduce bioavailability, and inhibitors or inducers of CYP3A4 and CYP1A2 can increase or decrease plasma concentrations.45 OAA can also interact with foods; for example, one paper reported that at least 15 OAA have clinically significant interactions with grapefruit.46
The complexity of OAA treatment can also include the cycling of medications, during which patients are required to have ‘on’ and ‘off’ days. For example, capecitabine for metastatic colorectal cancer is taken every 12 hours within 30 minutes of a meal for 2 weeks, followed by a 1-week break before a new cycle begins.47 One study demonstrated significant associations between regimen complexity and adherence when patients on simple regimens with the same dose and frequency throughout treatment were compared to those taking complex regimens that included alternating periods on and off medications and fluctuations in the prescribed dose.23 In the USA, the level of patient cost-sharing (where co-payments are required) has also been found to be a significant factor determining adherence to OAA, with some patients rationing medications or not having prescriptions processed. One study showed that treatment claims with cost-sharing >$500 were four-times more likely to be abandoned than claims with cost-sharing ≤100.48
A systematic review exploring adherence to OAA in breast cancer patients found that the most commonly reported motivator for improving adherence was the patient–provider relationship.49
In Germany, the DGOP recognised that community pharmacists have the potential to serve as agents for change in improving adherence to OAA therapy.
INITIATIVES SUPPORTING COMMUNITY PHARMACISTS WORKING WITH PATIENTS TAKING ORAL ANTICANCER AGENTS
In 2011, the DGOP joined forces with the German Cancer Society (Deutsche Krebsgesellschaft) to develop tools to support community pharmacists interacting with cancer patients taking OAA.
Key features of the Oral Anticancer Therapy – Safe and Effective initiative included provision of content for training programmes that are delivered by oncology pharmacy experts at regional meetings. The training programme consists of presentations developed by DGOP experts that are intended to be delivered across three separate sessions lasting a total of 8 hours:
- The first session, lasting 2 hours, covers the basics of cancer therapy, dealing with terminology, epidemiology, tumour development, and principles of cancer therapy.
- The second session, lasting 4.5 hours, covers applied oncology pharmacy and addresses individualisation of dose, adherence, and adverse drug effects and their management.
- The third session, lasting 1.5 hours, explores handling of OAA, storage, administration, handling of excreted materials, disposal of waste, and cleaning.
To date, around 3,000 German community pharmacists have attended educational sessions delivered around the country by 50 DGOP members (Klaus Meier, personal communication). In addition to organising training programmes, the DGOP has developed an online database providing key facts for the 72 OAA agents available in Germany. Community pharmacists can use these monographs both to educate themselves about the agents and to inform patients. The monographs are continually updated by a DGOP working group with the intention to further extend the monographs as more information about OAA becomes available online.
The online database can be used to provide each cancer patient with bespoke medication administration plans and a calendar to track drug administration so that the pharmacist can check for adherence when they refill medications. An important feature is the day-to-day record of wellbeing that contains a simple smiley face measurement survey showing five expressions ranging from happy (represented by a smiley face) to sad (represented by a crying face) that patients can use to document their condition on a daily basis (Figure 3). The pharmacist instructs patients that if they mark an unhappy face on two subsequent days they should make an urgent appointment to see their oncologist to adjust the dose and receive advice on how to avoid side effects. The DGOP recognise that it is important for patients to document how they feel daily, since it can be all too easy to forget adverse events when they see their oncologists. The initiative also offers the opportunity to collect information on thousands of patients taking OAA, which has the potential to gather side effect statistics that go beyond those available in drug trials. Ultimately, the DGOP hope to collect data for use in a study to demonstrate the effectiveness of good patient support by community pharmacists.
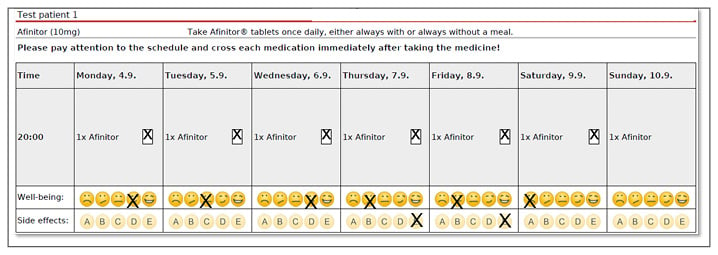
Figure 3: Screenshot of the online database developed by the German Society for Oncology Pharmacy (Deutsche Gesellschaft für Onkologische Pharmazie [DGOP]) as part of the Oral Anticancer Therapy – Safe and Effective initiative. Courtesy of Klaus Meier.
This shows a day-to-day record of patient wellbeing containing patient-specific information, with a calendar that patients can use to record taking their drugs, a ‘smiley face’ survey to show wellbeing, and opportunities to record different adverse events.
A: nausea/vomiting; B: skin reaction; C: mucositis; D: diarrhoea; E: exhaustion.
In 2015, the European Society of Oncology Pharmacy (ESOP) started the Empowering Patients to Improve Health Care for Oral Chemotherapy (EPIC) initiative, which extended the DGOP scheme to community pharmacists working in Slovenia and Estonia; training materials and drug information monographs were translated into the local languages. At the 4th European Conference of Oncology Pharmacy (ECOP), held from 25th–27th October 2018 in Nantes, France, the plan is to roll out the EPIC scheme to other European countries; Spain, France, Belgium, Austria, Denmark, Italy, Poland, Romania, and Bulgaria have already expressed an interest. Modifications to EPIC will undoubtedly be needed for implementation in countries that have different training protocols, modes of learning, and ways of dispensing OAA to Germany.
CONCLUSION
Many community pharmacists working with cancer patients taking OAA around the world have reported that they feel unsupported and require additional education. In Germany, the Oral Anticancer Therapy – Safe and Effective initiative has achieved great success, and it is hoped that when the programme is extended to other European countries it will lead to better support of cancer patients in the community and improved adherence to OAA with an overall impact on drug efficacy.








