Abstract
Patients indicate that among the most feared side effects of cancer are chemotherapy-induced nausea and vomiting (CINV), with up to 80% of patients affected if appropriate prophylaxis is not administered. CINV affects patient quality of life, may interfere with chemotherapy compliance which can possibly influence cancer survival outcomes, and results in greater healthcare resource utilisation. An array of antiemetics that act on different receptors involved in CINV pathways are available, as are antiemetic guidelines from various international and national bodies (such as the American Society of Clinical Oncology [ASCO], Multinational Association for Supportive Care in Cancer [MASCC] and European Society for Medical Oncology [ESMO], and National Comprehensive Cancer Network [NCCN]). Optimal management of CINV and other treatment-related side effects has been associated with improved quality of life, longer duration of anticancer treatments, and decreased utilisation of emergency care. Although progress has been made, there are still unmet needs, the greatest of which is the lack of complete nausea control.
INTRODUCTION
This review set out to identify the initiatives needed to achieve better cancer-induced nausea and vomiting (CINV) control worldwide. These include better adherence by healthcare professionals to evidence-based antiemetic guidelines and improved adherence by patients to antiemetic regimens. The use of netupitant with palonosetron (NEPA), a fixed combination therapy administered orally once per chemotherapy cycle, as well as mobile health (mHealth) apps may help with guideline adherence and patient compliance. Further developments will focus on the optimised use of new antiemetic compounds and alternative formulations to design simple and convenient regimens. In addition, as patient-specific risk factors are better defined, these can be incorporated into individualised regimens to obtain optimal antiemetic prophylaxis for that individual.
SEARCH STRATEGY
A literature search of PubMed was conducted in November 2019 without any date restrictions and using the following terms in various combinations: chemotherapy-induced nausea and vomiting, CINV, chemotherapy, nausea, vomiting, emesis, treatment, therapy, antiemetic, novel agent, NEPA, alternative treatment, alternative therapy/therapies, cost-effectiveness, economics, guideline, recommendation, control, developing country/countries, compliance, adherence, neurokinin 1 receptor antagonists (NK1RA), olanzapine, 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA), dexamethasone, dopamine receptor antagonist, cannabinoids, ginger, mobile technology, and risk factor. Papers that related to the aim of the study and were written in English were included. The reference lists of acquired papers were also searched for further relevant articles.
NEED FOR IMPROVED CONTROL OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING
The global incidence of cancer is on the rise; 17 million new cases and 9.6 million deaths occurred worldwide in 2018 and it is estimated there will be 27.5 million new cases of cancer each year by 2040.1 Patients with cancer experience many side effects while undergoing chemotherapy including alopecia, tiredness, and depression; however, the side effects most feared by patients are nausea and vomiting.2,3 Up to 80% of oncology patients may be affected by CINV if adequate prevention is not offered.4 CINV affects patient quality of life (QoL) including daily functioning, leisure activities, and the ability to eat and drink, and can result in complications including electrolyte imbalance, dehydration, and malnutrition.5 Importantly, CINV may also interfere with chemotherapy compliance or result in dose reduction and thus negatively influence cancer survival outcomes.6,7 In addition, emesis results in greater healthcare resource utilisation and higher cancer treatment costs as more inpatient, outpatient, and emergency department visits are required.8 Thus, CINV places a high burden on the individual, health services, and society.
Antiemetics are generally prescribed based on emetogenicity of the chemotherapy (high, moderate, low, or minimal) and type of CINV to be prevented. CINV can be acute or delayed, occurring 0–24 or 25–120 hours after chemotherapy, respectively. The incidence of delayed CINV varies depending on chemotherapy type, and it remains a challenge in terms of nausea control.9 CINV can also be anticipatory (a conditional response resulting from previous poor experience with chemotherapy), breakthrough (occurs despite the use of preventative drugs and may require rescue medication), and refractory (occurs after the unsuccessful use of antiemetics or rescue medications in the previous treatment cycle). As the different types of CINV are controlled through different pathways and neurotransmitters, various approaches for prevention and treatment are required.10
Optimal management of CINV and other treatment-related side effects has been associated with improved QoL, longer duration of anticancer treatments, and decreased utilisation of emergency care.10 Although progress has been made in the management of CINV, there are still unmet needs, the greatest of which is the lack of complete nausea control.11,12 Multiple initiatives are needed to achieve better control of CINV (Figure 1) and thus reduce the burden on the individual and society. These initiatives are discussed in detail below.
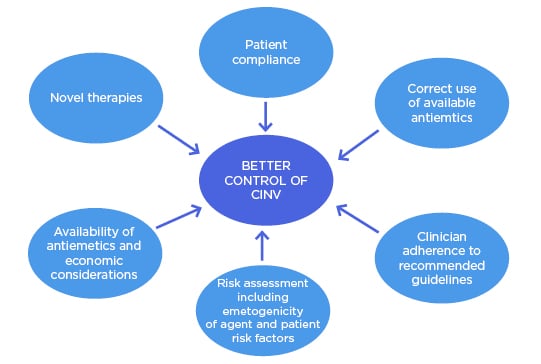
Figure 1: Initiatives to achieve better control of chemotherapy-induced nausea and vomiting.
CINV: chemotherapy-induced nausea and vomiting.
AVAILABLE ANTIEMETICS
An array of antiemetics that act on different receptors involved in CINV pathways is available. The correct use of these antiemetics can prevent CINV in 70–80% of patients.13 Antiemetic guidelines are available from international bodies such as the American Society of Clinical Oncology (ASCO) and the Multinational Association of Supportive Care in Cancer (MASCC) and the European Society of Medical Oncology (ESMO), and national bodies such as the National Comprehensive Cancer Network (NCCN).14-17 An overview of recommended antiemetics by emetogenic risk of chemotherapy and CINV type is displayed in Table 1.14-17
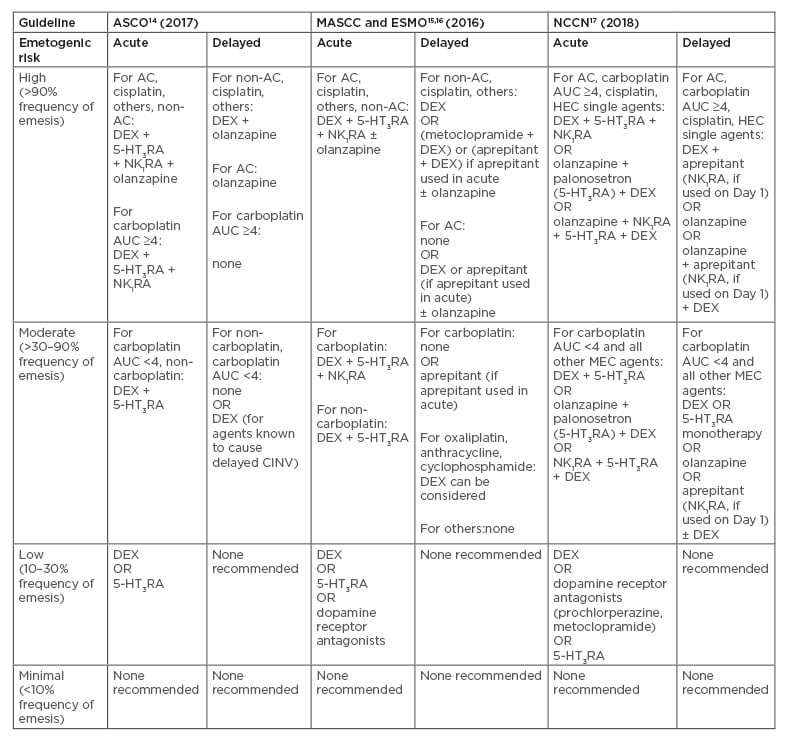
Table 1: Overview of recommended antiemetics by emetogenic risk of chemotherapy.14-17
Acute refers to 0–24 hours after chemotherapy, delayed refers to 25-120 hours after chemotherapy.
5-HT3RA: 5-HT3 receptor antagonist; AC: anthracycline and cyclophosphamide combination; ASCO: American Society of Clinical Oncology; AUC: area under the curve; CINV: chemotherapy-induced nausea and vomiting; DEX: dexamethasone; ESMO: European Society of Medical Oncology; HEC: highly emetogenic chemotherapy; MASCC: Multinational Association of Supportive Care in Cancer; MEC: moderately emetogenic chemotherapy; NCCN: National Comprehensive Cancer Network; NK1RA: neurokinin 1 receptor antagonist.
Neurokinin 1 Receptor Antagonists
The use of NK1RA has advanced antiemetic therapy over the last 15 years following the initial approval of oral aprepitant in 2003.18 As a result, this class of drugs is currently included in recommended guidelines. Several other NK1RA are now also available in many countries, including oral netupitant and intravenous (IV) fosnetupitant (available as fixed combinations with palonosetron), and oral rolapitant and IV fosaprepitant. The addition of a NK1RA to an antiemetic regimen can significantly reduce episodes of vomiting and the need for additional medications.19 Numerous randomised controlled trials (RCT) have shown a significantly greater rate of complete response (i.e., no emesis and no use of rescue medication) in patients on a three-drug regimen (i.e., NK1RA, 5-HT3RA, and dexamethasone) compared to those on a two-drug combination (i.e., 5-HT3RA and dexamethasone) in both acute and delayed CINV.20-28 However, most of these studies showed no significant improvement in delayed nausea control. Similarly, a network meta-analysis of the comparative effectiveness of NK1RA for highly emetogenic chemotherapy (HEC) concluded that NK1RA-containing regimens are associated with higher complete response rates than regimens without NK1RA.29
The cost effectiveness of the addition of NK1RA as antiemetics in patients with CINV has been demonstrated. A study from Hong Kong showed that the use of aprepitant compared to other drugs was associated with higher drug costs but a lower cost of emesis-related management. Thus, the aprepitant-containing regimen was deemed cost effective using the World Health Organization (WHO) cost-effectiveness threshold of three times gross domestic product per capita.30 A Japanese cost-utility analysis reported that the addition of aprepitant, but not fosaprepitant, to the 5-HT3RA and dexamethasone combination is cost effective.31 European studies have also demonstrated the cost effectiveness of aprepitant for CINV.32,33 A study that compared an aprepitant regimen (aprepitant, ondansetron, and dexamethasone) with a standard antiemetic regimen (ondansetron, dexamethasone, and metoclopramide) in patients with breast cancer in the UK reported that 78% of the cost of aprepitant was offset by reduced costs of healthcare resource utilisation. They reported an incremental cost-effectiveness ratio of £10,847/quality-adjusted life year with aprepitant compared to standard therapy; this is below the accepted UK threshold (£20,000–30,000/quality-adjusted life year) and suggests that aprepitant is cost effective for CINV prevention in UK patients with breast cancer.32 Similarly, a Belgian study confirmed that aprepitant-based CINV prevention was more effective and less costly than standard care.33
Despite their efficacy and cost effectiveness, NK1RA are underused in clinical practice. A retrospective study of administrative claims data from the USA showed almost universal use of dexamethasone and 5-HT3RA; however, only 60% and 80% of patients received a NK1RA in 2011 and 2016, respectively.18 Some institutions may limit the use of more expensive antiemetics by using a 5-HT3RA and dexamethasone combination in the first cycle and only add in a NK1RA if the patient experiences CINV.19 This practice is inconsistent with antiemetic guidelines for patients receiving HEC and for many receiving moderately emetogenic chemotherapy (MEC), and contradicts the fact that the first experience with chemotherapy is the most important in terms of CINV prevention. Patients who experience CINV during the first cycle are more likely to develop refractory or anticipatory CINV, whereas those whose CINV is controlled in the first cycle are more likely to do well in subsequent cycles.19,34 This highlights the importance of incorporating a NK1RA into the antiemetic regimen as per the recommended guidelines.
Netupitant with Palonosetron
Netupitant is currently only available in combination with palonosetron (a 5-HT3RA).18 NEPA represents the first antiemetic combination drug developed. Netupitant is a new, highly selective NK1RA while palonosetron has unique pharmacological and clinical characteristics compared to older 5-HT3RA as well as being more efficacious during the delayed CINV stage.35 NEPA has been shown to be safe, well tolerated, and highly effective over multiple HEC or MEC cycles.36 This antiemetic combination offers guideline-consistent prophylaxis by targeting two critical pathways associated with CINV in a single oral dose, administered only once per cycle.37 Development of this antiemetic combination offers a solution to the inconsistencies evident in guideline-consistent care (i.e., an absence of a NK1RA) for high-risk groups.38 Economic constraints, as well as complexity and inconvenience of the oral aprepitant regimen (e.g., 3 days aprepitant plus 1–3 days 5-HT3RA plus 1–4 days of dexamethasone), may also have played a role in the underutilisation of NK1RA.37,38
Cost-effectiveness analyses have shown benefit of NEPA over other drug combinations.39,40 Botteman et al.,41 who conducted the first economic analysis comparing NK1RA regimens, reported that NEPA was more cost effective than an aprepitant-based regimen in patients undergoing HEC. In addition, NEPA with dexamethasone is not only superior to palonosetron with dexamethasone in preventing acute and delayed CINV following MEC and HEC, but it is also more cost effective.42,43 A real-life study has confirmed the benefit of NEPA seen in clinical trials; 630 patients were enrolled in a prospective, multicentre, noninterventional study where NEPA was effective in the prevention of CINV in patients who received carboplatin- and oxaliplatin-based MEC and QoL, and they remained stable during the course of chemotherapy.44 Advantages and disadvantages of all antiemetics available for CINV are displayed in Table 2.
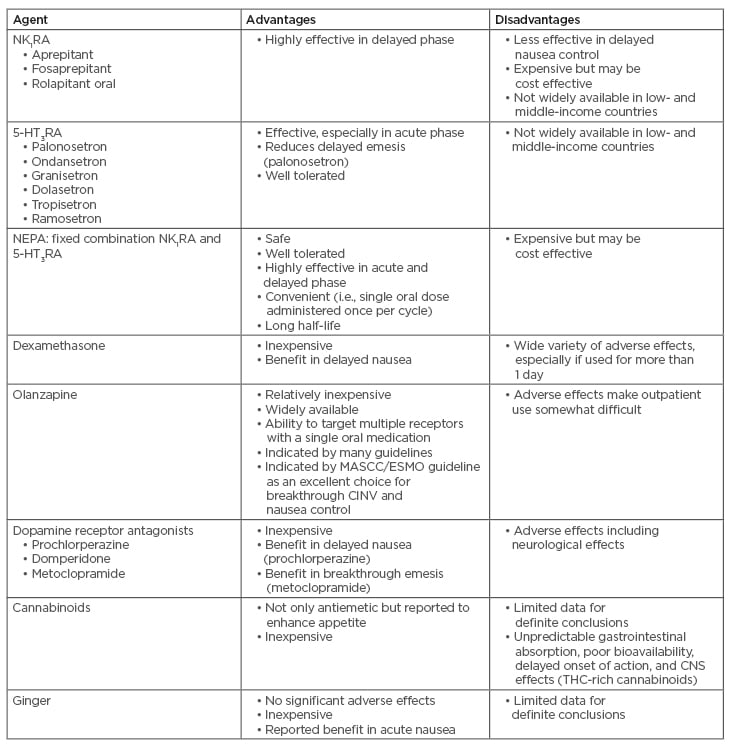
Table 2: Advantages and disadvantages of antiemetics available for chemotherapy-induced nausea and vomiting.
5-HT3RA: 5-HT3 receptor antagonist; CINV: chemotherapy-induced nausea and vomiting; CNS: central nervous system; ESMO: European Society of Medical Oncology; MASCC: Multinational Association of Supportive Care in Cancer; NEPA: netupitant and palonosetron; NK1RA: neurokinin 1 receptor antagonist; THC: tetrahydrocannabinol.
CLINICIAN ADHERENCE TO RECOMMENDED GUIDELINES
Guidelines recommended by ASCO, MASCC and ESMO, and NCCN are based on the consensus opinions of international experts and most recent clinical trial data, and are regularly updated when new data become available. Despite being available and accessible, in many instances these guidelines are not followed. Very low adherence to antiemetic guidelines has been reported in clinical practice in the European Union (EU); only 16% of all patients on HEC or carboplatin-based regimens received the recommended combination of a NK1RA, 5-HT3RA, and dexamethasone.45 In addition, 17% of patients on HEC or MEC received no antiemetics at all.45 A further European study of >200 oncology nurses revealed that guideline awareness was generally low; less than one-half of nurses surveyed were familiar with the ASCO (46%) and MASCC and ESMO (40%) guidelines.46 Key discrepancies between guideline recommendations and clinical use included underutilisation of the recommended NK1RA plus 5-HT3RA plus steroid combination on Day 1 (55%), and high use of 5-HT3RA (50%) on Days 2–5 when a steroid (63% use) is recommended for patients on HEC. Use of metoclopramide was also high in patients on both HEC and MEC, with approximately 30% and approximately 50% reporting use on Day 1 and Days 2–5, respectively.46 Reasons for poor guideline adherence may have included the complexity of prophylactic treatment for HEC, mucositis, and depression in patients with cancer that may have affected compliance, concurrent use of multiple medications, physician workload, and suboptimal communication between provider and patient.18 Oncologists reported usage of weaker antiemetic regimens than necessary, underestimation of chemotherapy emetogenicity, and patient nonadherence because of administration errors or missed/delayed doses to be the main reasons for antiemetic treatment failure.47
Following the recommended guidelines for CINV has been shown to improve patient outcome and reduce associated costs. A prospective, observational, multicentre study that enrolled chemotherapy-naïve adults initiating HEC or MEC for cancer showed that the guideline-consistent CINV prophylaxis cohort was 43% more likely than the guideline-inconsistent prophylaxis cohort to achieve a complete response (i.e., no emesis and no use of rescue medication) during the 120 hours after the first chemotherapy cycle.38 Similarly, a Turkish study of 100 chemotherapy-naïve patients showed significant differences in complete control (i.e., no emetic episodes, rescue therapy, or nausea) and Functional Living Index Emesis (FLIE) score between the guideline (i.e., MASCC and ESMO 2014)-adherent and guideline-nonadherent groups.48 The latter had a higher incidence of diarrhoea, headache, swallowing difficulties, and dark-coloured stool. Thus, clinicians and healthcare professionals are strongly encouraged to adhere to recommended guidelines to improve CINV control.
Antiemetic guidelines are valid in countries where drugs are available and reimbursed; however, this is not the case in some parts of the world. Bevoor et al.49 conducted a study to look at utilisation patterns of antiemetics to control CINV with respect to standard international recommendations in 316 patients treated for cancer in India.
They found numerous discrepancies including the prescription of ondansetron 32 mg, despite this not being recommended in guidelines; palonosetron 0.25 mg given for 3 days continuously rather than only on the first day of chemotherapy; dexamethasone given at a dose of 20 mg in many patients rather than 8–12 mg; use of promethazine and wide use of metoclopramide which is not recommended in guidelines; and limited use of NK1RA because of patient affordability and unwillingness of government insurance to fund the drug. Similarly, most Thai patients with cancer receiving HEC do not have access to NK1RA and palonosetron because Thailand is a limited-resource country, despite international guidelines recommending the use of NK1RA, 5-HT3RA, and corticosteroid for prevention of CINV after HEC.50 The use of olanzapine, which is 70% less costly than aprepitant, instead of a NK1RA in combination with a 5-HT3RA and dexamethasone, has been considered as an alternative option.51 An RCT found olanzapine to be as effective as aprepitant, both in combination with IV palonosetron and dexamethasone in terms of complete response in patients receiving HEC.52 Furthermore, olanzapine has shown superior efficacy over both NK1RA in nausea prevention and oral aprepitant in the delayed phase of CINV and in nausea control.53,54 Further guidance is needed for those who cannot afford the antiemetic regimens recommended in current guidelines.
PATIENT FACTORS
Patient factors, such as younger age, female sex, low alcohol intake, anxiety, and a history of motion sickness or nausea during pregnancy, may also increase risk of CINV.55,56 However, these factors have not yet been incorporated into formal risk assessment, and treatment choice/recommendations are still largely based on emetogenicity of the chemotherapeutic agent.19 An algorithm has recently been developed to identify patients at high risk of CINV before each chemotherapy cycle.57 Clinical application of this tool would allow patient risk factors to influence the selection of optimal antiemetic prophylaxis for that individual. In addition, use of drugs like NEPA, which is administered in a single oral dose once per cycle, will help to improve patient compliance and thus achieve better CINV control.37
Patient compliance can be further improved by novel innovations such as mHealth, which is the use of mobile devices for healthcare delivery. There has been a recent boom in mobile technologies for the self-management of chronic diseases.58 mHealth has the potential to improve patient management of symptoms and compliance, reducing subsequent hospital visits and burden of disease. Less decline in health-related QoL and better overall survival was evident in patients with cancer who self-reported symptoms via web-based Symptom Tracking and Reporting (STAR), compared to patients who discussed symptoms only during clinical encounters with their oncologist or via telephone in the case of concerning symptoms.59 A further study demonstrated improved survival in patients with advanced-stage lung cancer who self-reported symptoms using a web-mediated follow-up algorithm compared to patients who underwent routine follow-up with CT scans every 3–6 months.60 However, despite its potential, evidence on the actual use of mobile technologies in cancer care is not promising. A recent cross-sectional, web-based survey across the USA and five European countries reported a much lower proportion of patients with cancer (28.46%) using mHealth than clinicians (76.97%).61 Patient concerns regarding use of mHealth included a preference for traditional means of communication with their doctor, lack of knowledge regarding the potential of information technology, and doubt about the reliability and effectiveness of mHealth for medical purposes. For mHealth to be effective, clinician and patient usage rates need to converge. Ideally, cancer apps should be designed to strengthen the patient–physician relationship and to ease physicians’ workload, as well as being tested for validity and effectiveness and fit the criteria for reimbursement.61 Further studies are currently underway, including an RCT to evaluate a mobile supportive care app for patients with metastatic lung cancer.62
NOVEL THERAPIES
Despite progress, a significant proportion of patients still experience nausea and vomiting while receiving optimal treatment. Novel therapies, especially for the control of nausea, are still needed, with the ultimate goal of complete CINV control. BarhemsysÒ (formerly ADP421), a selective dopamine antagonist, has completed Phase III clinical development for the prophylaxis and treatment of postoperative nausea and vomiting (PONV).63 The U.S. Food and Drug Administration (FDA) has given the following label: indicated in adults for 1) prevention of PONV, either alone or in combination with an antiemetic of a different class; and 2) treatment of PONV in patients who have received antiemetic prophylaxis with an agent of a different class or have not received prophylaxis.64 Its role in CINV has yet to be determined. Several organisations (ASCO, ESMO, NCCN, and the Institute for Clinical and Economic Review [ICER]) have developed frameworks to assess the value of new cancer drugs to help clinicians, patients, and payers make decisions about treatments.65 These frameworks differ in methods and approaches; however, they all help to promote a wider culture of allocative efficiency and prioritisation.66
ALTERNATIVE THERAPIES
Alternative therapies have been used and tested for the control of CINV, including marijuana leaf extract, ginger, acupuncture, Chinese herbal medicine, and music therapy.67-69 Most studies that assessed the use of smoked marijuana or synthetic oral tetrahydrocannabinol medicines (THC; dronabinol, nabilone) in CINV have shown limited efficacy, were not sufficiently powered, and used outdated control antiemetic arms.70 A small, pilot, double-blind, randomised trial of 16 patients with CINV after MEC, despite prophylaxis with a guideline-consistent antiemetic regimen, showed nabiximols, a THC/cannabidiol cannabis extract derived from the Cannabis sativa plant containing THC and cannabidiol in defined and near-equal amounts, to have substantial efficacy, high acceptability by patients, and manageable side effects for the secondary prevention of CINV.71 Early, though small, trials have shown THC to also improve appetite in these patients, with positive implications for overall performance status.68 There is also some evidence to suggest ginger may help to prevent acute emesis. An RCT in which ginger was added to a 5-HT3RA plus dexamethasone combination showed reduction in acute, but not delayed, emesis.72 While these alternative therapies have been reported to be of help, further research is needed as there are insufficient data to draw any definitive conclusion.
CONCLUSION
Multiple initiatives are needed to achieve better control of CINV to minimise the negative impact on patients with cancer, improve patient outcomes, and reduce costs. Improved adherence to international antiemetic guidelines by healthcare professionals and compliance to antiemetic regimens by patients are both critical. NEPA may contribute to this improvement, as it is a fixed combination administered orally only once per chemotherapy cycle, as may the use of mHealth. Further developments will focus on the optimised use of new antiemetic compounds and alternative formulations to design simple and convenient regimens that ensure guideline adherence and patient compliance. Given the scarcity of resources, treatments must be assessed holistically, including all costs and benefits that accrue to the patient, healthcare system, and caregivers. The key question for decision makers, including medical doctors, in times of limited resources has shifted from ‘does it work?’ to ‘is it worth it?’. As patient-specific risk factors are becoming better defined, these can be incorporated into individualised regimens to obtain optimal antiemetic prophylaxis for that individual. Further research should also focus on using real-world data to confirm existing regimens or to develop alternative improved regimens for better CINV control.

![EMJ Oncology 8 [Supplement 1] Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/08/EMJ-Oncology-8-Supplement-1-Feature-Image-940x563.jpg)






