Abstract
Background: Epithelial myoepithelial carcinoma (EMC) is a rare biphasic tumour of the salivary gland with two cell types of inner ductal cells and outer layer of clear cells. In the literature, there are only a few reports of EMC originating from the hard palate.
Case report: A 58-year-old female presented to the authors’ institution with partially submucosal lesion in the posterior aspect of the hard palate on the left side for 1 month. Biopsy was suggestive of a multinodular tumour with round to oval cells and a moderate number of pale eosinophilic to clear cytoplasm and round to oval, centrally to eccentrically placed, mildly pleomorphic vesicular nuclei suggestive of EMC of the hard palate. Immunohistochemically, cytokeratin (CK 5/6) showed strong cytoplasmic positivity highlighting the luminal epithelial cells. The myoepithelial cells showed strong nuclear positivity for p63 and cytoplasmic positivity for calponin. The patient underwent surgical resection of the tumour with a local flap cover and split skin graft and all the margins were negative in the final histopathological examination with erosion of the underlying bone. The patient was kept under observation and has been free of the disease for the past 12 months.
Conclusion: Diagnosis of EMC is rare and is to be kept as a differential diagnosis during the evaluation of minor salivary gland tumours of palate.
BACKGROUND
Epithelial myoepithelial carcinoma (EMC) was initially described by Donath et al.1 and was previously referred to with various terminologies such as adeno-myoepithelioma, clear cell adenoma, or carcinoma. EMC is a rare biphasic tumour of the salivary gland with two cell types: an inner layer of duct lining cells and an outer layer of clear cells, which typically form double-layered duct-like structures.2 The tumour has a relatively low incidence, accounting for <1% of malignant salivary gland tumours.3,4 This tumour arises most frequently in the parotid gland (80%), but lesions have also been reported in the submandibular glands (10%) and minor salivary glands (1%).5 In the minor glands of the oral mucosa, EMC represents approximately 5% of all salivary gland tumours.6 Only a few case reports of EMC originating from the hard palate are present in the literature.7-17 The authors herewith report this case for its rarity of the tumour in the hard palate.
CASE REPORT
A 58-year-old female presented to the oncology outpatient department with the complaint of ulcerative lesion in the upper palate of 1-month duration which was progressively increasing in size and was associated with occasional pain. Examination revealed a partially ulcerated submucosal lesion in the posterior aspect of the left side of the hard palate, 15 mm in front of the hard palate–soft palate junction, and not extending to the midline (Figure 1).
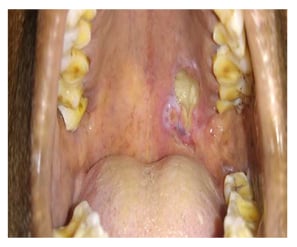
Figure 1: Clinical picture showing submucosal partially ulcerative lesion in the posterior aspect of the left side of the hard palate.
There were no significant palpable neck nodes. CT imaging identified thickening of the mucosa with underlying bone erosion, and there were no nodal metastases. The chest roentgenogram was normal.
Intraoral biopsy was performed, and the histopathological sections showed multinodular tumour with tumour cells arranged in an organoid fashion. The tumour was made up of round to oval cells with a moderate amount of pale eosinophilic to clear cytoplasm and round to oval, centrally to eccentrically placed, mildly pleomorphic vesicular nuclei, with few showing small prominent nucleoli. Focally luminal spaces lined by flattened cells were also noted, which were suggestive of EMC of the hard palate (Figures 2a and 2b). A diagnosis of EMC with predominance of myoepithelial clear cells was suggested. Immunohistochemistry was carried out to rule out other clear cell tumours of the salivary gland. Immunohistochemically, cytokeratin (CK 5/6) showed strong cytoplasmic positivity highlighting the luminal epithelial cells. The myoepithelial cells showed strong nuclear positivity for p63 and cytoplasmic positivity for calponin; however, staining for smooth muscle actin was negative (Figures 3a and 3b). Thus, the biphasic nature of the tumour was confirmed, and a diagnosis of EMC was established.
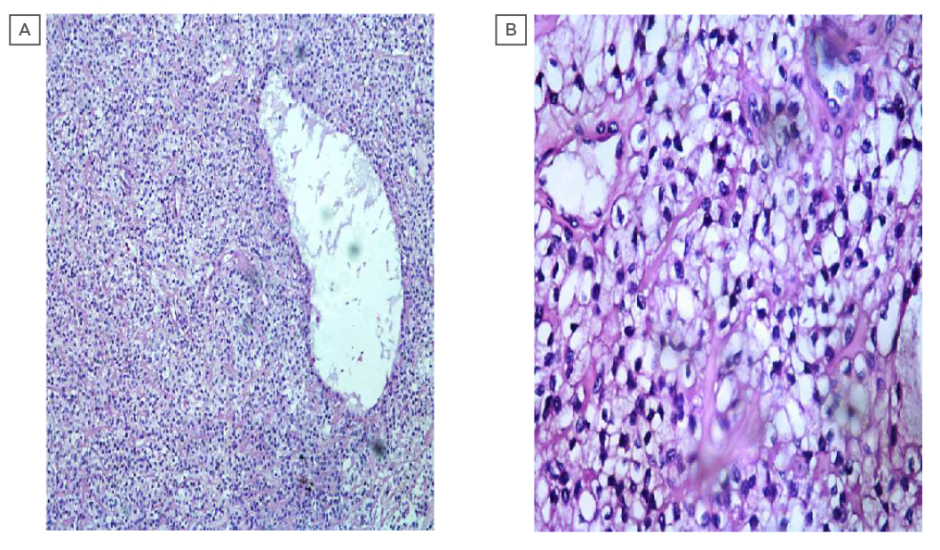
Figure 2: (A) photomicrograph showing epithelial component featuring duct like structures surrounded by clear myoepithelial cells (100X; haemotoxylin and eosin stain). (B) photomicrograph showing epithelial component featuring duct like structures lined by a single layer of cuboidal epithelium surrounded by clear myoepithelial cells.
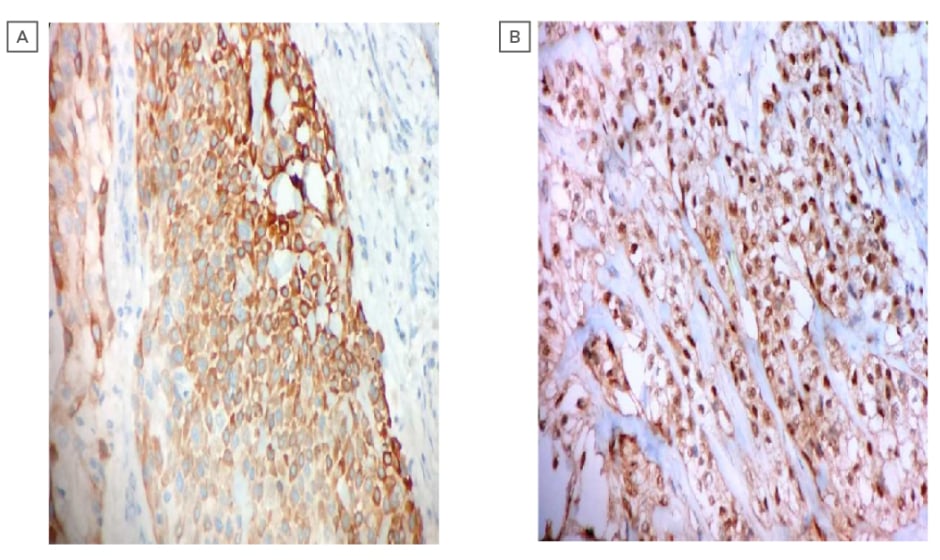
Figure 3: (A) photomicrograph showing the immunohistochemistry of strong cytoplasmic positivity of cytokeratin (CK 5/6) (400X; haemotoxylin and eosin stain). (B) photomicrograph showing the immunohistochemistry of strong nuclear positivity of P63 (400X, haematoxylin and eosin stain).
The patient underwent surgical resection of the tumour through modified Weber–Ferguson incision and partial maxillectomy was conducted with 10 mm gross margins, with the frozen section of the margins being negative (Figure 2a). The reconstruction of the tumour was performed with local flap cover and split skin graft. The postoperative course of the patient was normal. The final histopathological report suggested that the tumour, with a size of 30×15 mm, and the microscopic and immunohistochemistry features were the same as the preoperative biopsy. All the margins were free of tumour and the bone was eroded by the tumour. After discussion in the multispeciality tumour board clinic, the patient was given the option of observation or adjuvant radiation and the patient opted for observation. The patient has been free of the disease for the past 12 months.
DISCUSSION
EMC was defined as a solitary pathological
diagnosis in 1991 by the World Health Organization (WHO).18 The mean age of EMC patients is approximately 60 years and it affects females at a ratio of 1.5:1.0 with a mean tumour size of approximately 29 mm.19 EMC is a low-grade malignancy, and high-grade or dedifferentiated EMC cases have rarely been reported. Some morphologically low-grade myoepithelial carcinomas behave aggressively. EMC is a malignant biphasic salivary-type tumour and the diagnosis is based on conventional light microscopy, confirmed by immunohistochemical and ultrastructural investigation. Histopathologically, the tumour is characterised by well-defined tubules with two cell types: an outer layer of myoepithelial cells with clear cytoplasm surrounding an inner lining of eosinophilic cuboidal epithelial cells resembling intercalated ducts.20 In an observation made by Aydil et al.,21 the most common malignancies in the hard palate are minor salivary gland tumours (60.6%), followed by benign mesenchymal tumours (15.2%), squamous cell carcinoma (12.1%), malignant melanomas (6.1%), lymphomas (3.0%), and sarcomas (3.0%).
Immunohistochemical diagnosis involves various criteria and studies have commonly reported positivity for epithelial markers including CK 7, 14, and 5/6; S100 protein; endothelial membrane antigen; and smooth muscle actin. Calponin and glial fibrillary acidic protein have also been reported to be sensitive markers of myoepithelial differentiation in salivary lesions. Furthermore, p63 has also recently become a widely-used marker for abluminal cells, in both basal and myoepithelial, showing nuclear immunoreactivity.20 The tumour in this case was positive for p63 and calponin which helped in the conformation of the diagnosis. Recent molecular studies with PLAG1 and HMGA2 cellular rearrangements showed that 80% of EMCA arise from pleomorphic adenoma.22
EMC of the minor salivary glands are very rare and only a few cases have been reported with palatal origin.7-17 Most of the reported cases had painless and well-circumscribed masses associated with surface ulceration. There is no consensus as to the treatment of minor salivary gland EMC as the number of patients is too small to allow for controlled treatment trials. Tumour resection with negative surgical margin is the primary modality of the treatment. The role of adjuvant chemotherapy in EMC is not well documented. Seethala et al.19 found that the recurrence rate of EMC was 36.3%, and survival rates were 93.5% and 81.8% for 5 and 10 years, respectively.
CONCLUSION
In conclusion, diagnosis of EMC is rare and is to be borne in mind as a differential diagnosis during the evaluation of minor salivary gland tumours of the palate. The difficulty in establishing the pathological diagnosis implies the need for experienced pathologists and knowledge of immunohistochemical evaluation.