Speakers: Martin Reck,1 Takashi Kohno,2 Nobuyuki Takakura3
- Department of Thoracic Oncology, Lung Clinic Grosshansdorf, Germany
- Division of Genome Biology, National Cancer Center Research Institute, Tokyo, Japan
- Immunology Frontier Research Center, Osaka University, Japan
Support: The publication of this post was funded by Eli Lilly.
Disclosures: Reck has received honoraria for lectures and consultancy from AbbVie, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Lilly, Merck, Mirati, MSD, Novartis, Pfizer, and Roche. Kohno has received research funds from Sysmex Corporation and Chugai; and has taken advisory roles with Eli Lilly Japan and LOXO Oncology. Takakura has received honoraria from Eli Lilly Japan.
Acknowledgements: Medical writing support was provided by Subhashini Muralidharan of inScience Communications, London, UK.
Key takeaways
The two most common EGFR mutations (the exon 19 deletion and the exon 21 [L858R] mutation) differ in their kinase domain structures at different sites, resulting in differential sensitivity to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs).1-7
Anti-angiogenic agents normalise tumour vasculature, resulting in improvement of EGFR-TKI uptake by tumour tissue, when used in combination therapy.8,9 This could be critical for patients with exon 21 (L858R), whose tumours are less responsive to EGFR-TKI monotherapy.7,10
The RELAY trial results show that combination therapy with EGFR-TKI plus an anti-angiogenic agent can improve clinical outcomes, in both exon 19 Del and the exon 21 (L858R) mutation.11 RELAY is an effective treatment option especially for patients with exon 21 (L858R) mutation.11
BACKGROUND
EGFR gene mutations have been reported in 15–60% of non-small cell lung cancers (NSCLCs) with an important difference in prevalence between Eastern and Western populations.1,2 The most common EGFR-activating mutations in NSCLC include in-frame deletions in exon 19 and a substitution in exon 21 (L858R) which account for 47% and 41%, respectively, of observed EGFR mutations in NSCLC.1 Currently, EGFR-TKIs (tyrosine kinase inhibitors) are the treatment of choice for EGFR-mutated tumours, but the treatment is not tailored to the specific exon subtype.
EGFR-TKIs have significantly improved efficacy over systemic chemotherapy as first-line therapy. Median progression-free survival (mPFS) with systemic chemotherapy is about 5 months, whereas mPFS values between 9.2 and 18.9 months have been reported for monotherapy with first, second, and third generation TKIs.13-24 The current standard of care in first-line EGFR-mutated NSCLC is osimertinib, with a mPFS of 18.9 months in the intention-to-treat population, reported in the Phase III clinical trial, FLAURA.13 Nevertheless, subgroup analyses point to a stark difference between patients with different EGFR mutations: in patients with exon 19 deletion (Del), the reported mPFS was 21.4 months, while in patients with exon 21 (L858R) mutation subtype, the reported mPFS was 14.4 months.13
Here the authors aim to highlight the differences between the most common EGFR subtypes and the clinical implications as well as discuss how we can improve outcomes for patients with the exon 21 (L858R) mutation subtype.
WHAT ARE THE BIOLOGICAL DIFFERENCES BETWEEN EXON 19 DEL AND EXON 21 (L858R) AND HOW DO THEY DIFFERENTIALLY AFFECT TREATMENT OUTCOMES?
The exon 19 Del and exon 21 (L858R) mutations alter the conformation of the kinase domain of the EGFR protein. The wild-type EGFR protein switches between the active and inactive conformations (Figure 1).2 Molecular modelling and crystallographic studies suggest that the exon 19 Del and the exon 21 (L858R) mutations destabilise the inactive conformation of EGFR.1 This leads to increased receptor dimerisation and increased kinase activity, when compared with the wild-type EGFR protein.1 However, the stability of the active form differs between the two mutations: the αC-helix of exon 19 Del has an inward conformation with limited movement, making it stable in the active form.2
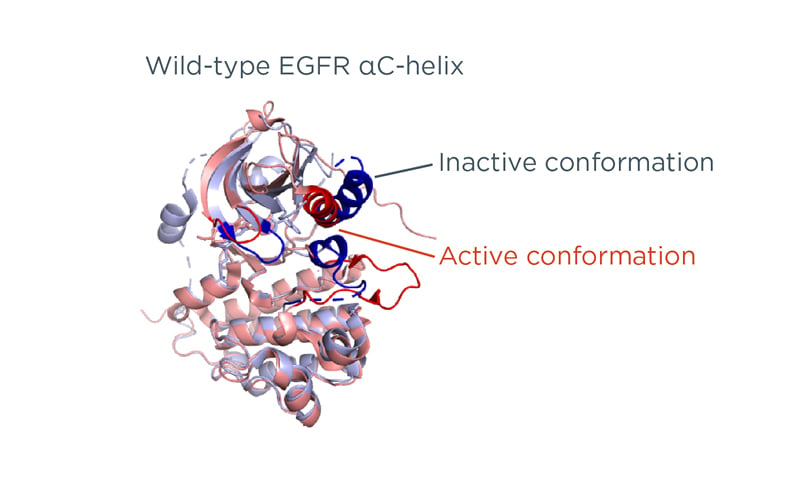
Figure 1: Structure of the wild-type epidermal growth factor receptor protein based on protein data bank structure 1XKK and 1M17.
Personal contribution from Kohno.
EGFR: epidermal growth factor receptor.
On the other hand, the leucine residue forms hydrophobic interactions that stabilise the inactive conformation; when it is replaced by a positively charged arginine, the hydrophobic interactions are disrupted, causing instability of the inactive form.3 Consequently, the L858R mutated EGFR protein takes the active conformation more easily than the inactive conformation.3 Because the exon 19 Del has an inward structure of the αC-helix due to the physical change caused by the lack of amino acids, its stability is presumed to be greater than that of exon 21 (L858R).2
This difference in the effect on EGFR protein structure is key to understanding the biochemical differences between these mutation subtypes. Because EGFR-TKIs bind to the kinase domain when it is in the active conformation4, mutations that stabilise EGFR in the active conformation increase its sensitivity to TKIs. Exon 19 mutated EGFR binds to EGFR-TKIs more easily than exon 21 (L858R) mutated EGFR, and can therefore be inhibited at a lower dose of the EGFR-TKI (Figure 2).5-7
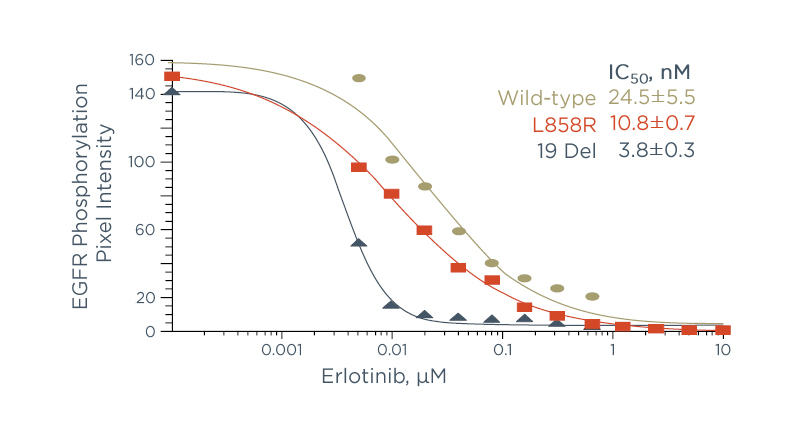
Figure 2: A higher dose of erlotinib is needed to inhibit exon 21 (L858R) than exon 19 deletion.19
The Scientific Perspective
Takashi Kohno stated: “Exon 19 Del mutated EGFR binds more easily to EGFR-TKIs than exon 21 (L858R) mutated EGFR, therefore, a lower dose is sufficient to inhibit its activity. This translates into an increased responsiveness to therapy. Studies have shown that even though exon 19 Del mutated tumours are more malignant than exon 21 (L858R) mutated tumours, they are also more sensitive to EGFR-TKIs.7,10 Therefore, monotherapy with EGFR-TKIs works better in patients with exon 19 Del, than in those with exon 21 (L858R).”
This difference in outcomes highlights an unmet need in the treatment landscape for EGFR-mutated NSCLC. One way of meeting this need is by improving the uptake of EGFR-TKIs by targeting VEGFR pathways with an anti-angiogenic agent.
WHAT IS THE RATIONALE BEHIND COMBINING EPIDERMAL GROWTH FACTOR RECEPTOR-TYROSINE KINASE INHIBITORS WITH AN ANTI-ANGIOGENIC AGENT?
VEGF-mediated signalling plays a key role in blood vessel formation and tumorigenesis.25 High levels of VEGF in tumours result in an abnormal tumour vasculature.26 Tumour blood vessels have abnormal perfusion and are leaky, resulting in increased interstitial fluid pressure.27 This in turn reduces the difference in fluid pressure between the tumour and the blood vessel, reducing the transport of drugs into the tumour.28 Anti-angiogenic agents have been shown to reconstitute the tumour vasculature (Figure 3A). In a recent study, Tsukada and colleagues transplanted patient-derived tumour tissue into mice and observed in vivo changes to tumour blood vessels after treatment with ramucirumab or isotype IgG.8 Ramucirumab treatment significantly reduced the length of human tumour blood vessels (Figure 3B) and tended to induce normalisation of vasculature, compared with isotype IgG.8 In a separate study, Chatterjee et al. showed that short-term pre-treatment with an anti-angiogenic agent induced a transient period of improved blood flow, associated with reduced leakiness from tumour vessels.9 EGFR-TKI treatment begun during this period improved EGFR-TKI uptake by tumour tissue (Figure 3C), which reduced tumour volumes significantly, compared with EGFR-TKI treatment without anti-angiogenic pre-treatment.9
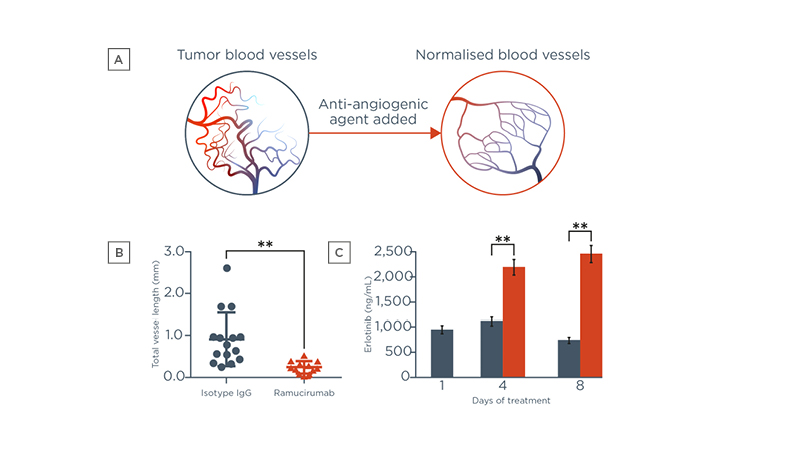
Figure 3: Addition of an anti-angiogenic agent (A and B) normalises tumour blood vessels and (C) improves erlotinib uptake.24,26,27
The Scientific Perspective
Nobuyuki Takakura stated: “Pre-treatment with an anti-angiogenic agent normalises the tumour blood vessels, which in turn lowers the interstitial fluid pressure and restores normal blood flow. This allows for increased uptake of EGFR-TKIs by the tumour, and leads to a significant reduction in tumour volume. Therefore, normalising tumour vasculature is key to increasing the effectiveness of EGFR-TKIs.”
HOW CAN WE IMPROVE TREATMENT OUTCOMES FOR PATIENTS WITH THE EXON 21 (L858R) MUTATION?
EGFR-TKI monotherapy results in poorer prognosis for patients with the exon 21 (L858R) mutation than for those with the exon 19 Del. In a meta-analysis of six randomised, controlled trials, patients with exon 21 (L858R) had a lower probability of PFS than those with the exon 19 Del, when undergoing EGFR-TKI monotherapy.29 Patients with exon 21 (L858R) mutation were found to have a higher hazard ratio (hazard ratio [HR]: 0.49 [95% confidence interval [CI]: 0.40, 0.60]) than exon 19 Del patients (0.28 [95% CI: 0.23, 0.34]) for the effect of treatment with EGFR-TKI monotherapy versus chemotherapy (Figure 4).29
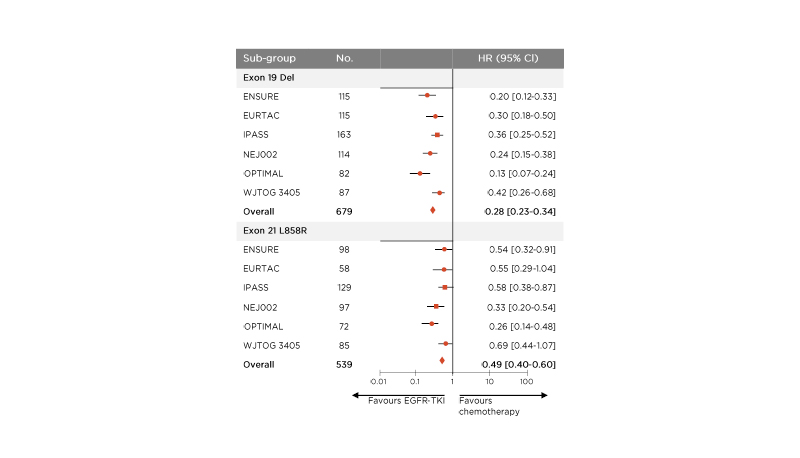
Figure 4: Patients with the exon 21 (L858R) mutation have a higher hazard ratio with epidermal growth factor receptor-tyrosine kinase inhibitor therapy versus chemotherapy than patients with the exon 19 deletion with monotherapy.28
CI: confidence interval; Del: deletion; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor; HR: hazard ratio; No.: number.
In clinical trials, EGFR-TKI monotherapy, irrespective of the use of first, second, or third generation TKIs, results in poorer prognosis for patients with the exon 21 (L858R) mutation, than for patients with the exon 19 Del (Figure 5), with differences in mPFS up to 7 months.13,14,16,17
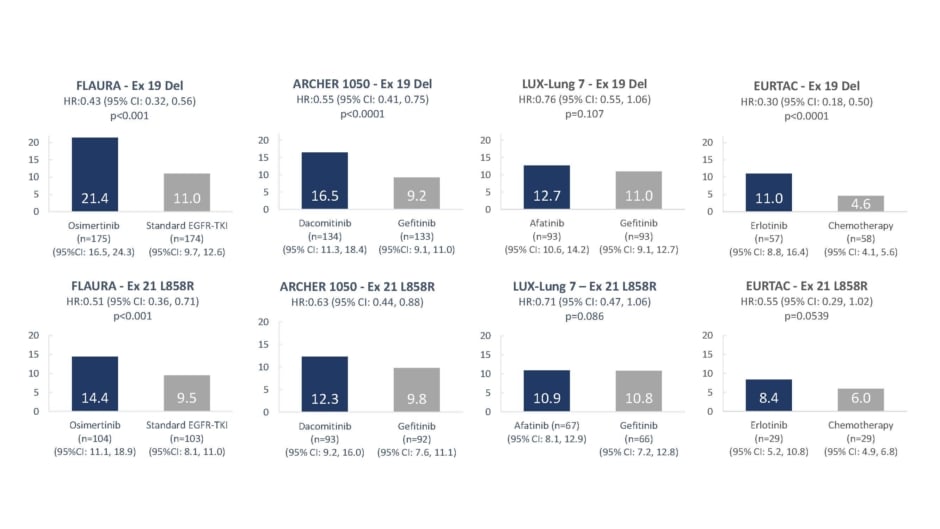
Figure 5: Median progression-free survival values with epidermal growth factor receptor-tyrosine kinase inhibitor monotherapy by mutation sub-type.3,4,6,7
CI: confidence interval; Del: deletion; HR: hazard ratio.
One strategy to extend PFS and improve tumour control is dual blockade of the EGFR and VEGF pathways, which is supported by preclinical and clinical evidence.8,9,11,30-32 The RELAY trial assessed the efficacy and safety of dual blockade using an EGFR-TKI plus/minus ramucirumab, which is a human IgG1 VEGFR2 antagonist.29 The study enrolled 449 patients from 13 countries.11 Patients received oral erlotinib 150 mg/day and either intravenous ramucirumab 10 mg/kg or intravenous placebo once every 2 weeks.11 The primary endpoint was investigator-assessed PFS in the intention-to-treat population.11 At a median follow-up of 20.7 months (interquartile range: 15.8–27.2), mPFS for ramucirumab plus erlotinib was 19.4 months (95% CI: 15.4, 21.6) versus 12.4 months (95% CI: 11.0, 13.5) for placebo plus erlotinib.11
The EGFR mutation subgroups had similar mPFS benefit as the overall included population. Patients with exon 19 Del had a mPFS of 19.6 months (95% CI: 15.1, 22.2) with combination therapy, versus 12.5 months (95% CI: 11.1, 15.3) with monotherapy (Figure 6).11 Those with the exon 21 (L858R) mutation had a mPFS of 19.4 months (95% CI: 14.1, 21.9) with combination therapy, versus 11.2 months (95% CI: 9.6, 13.8) with monotherapy (Figure 6).11As expected, the addition of ramucirumab to erlotinib increased the incidence of several adverse events when compared with erlotinib plus placebo; however, these treatment-emergent adverse events were Grade 1 –2, except for hypertension.11 Overall, adding ramucirumab to erlotinib did not negatively affect the patients’ ability to continue study treatment: discontinuation of study treatment due to TEAEs occurred in 13% of patients in the combination therapy group versus 11% of patients in the monotherapy group.11
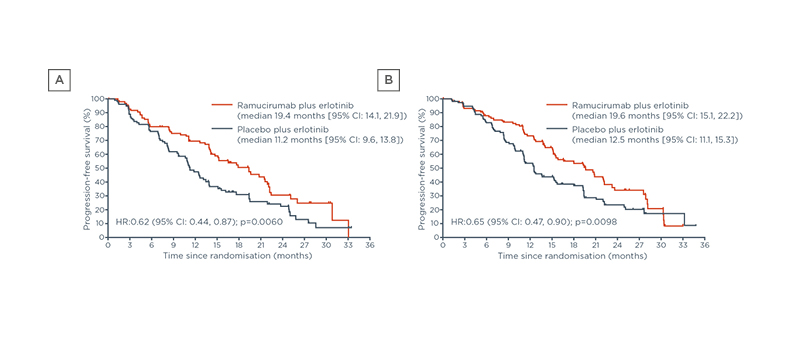
Figure 6: The RELAY regimen provides similar progression-free survival benefits for (A) patients with exon 19 deletion and (B) patients with the exon 21 L858R mutation.29
CI: confidence interval; HR: hazard ratio.
Thus, the combination of EGFR-TKI and anti-angiogenic agent improves PFS in the overall included population compared with EGFR-TKI monotherapy.11,30–32 As patients with the exon 21 (L858R) mutation have historically had a poorer prognosis in response to EGFR-TKI monotherapies, this subgroup may be a higher priority for EGFR-TKI and anti-angiogenic combination therapy.
The Clinician’s Perspective
Martin Reck stated: “Evidence suggests that dual blockade of the VEGFR and EGFR pathways is more effective at inhibiting tumour growth than either approach alone in EGFR-mutant metastatic NSCLC. Subgroup analyses show similar PFS benefit between exon 19 Del and exon 21 (L858R) mutation subtypes for the combination treatment. Dual blockade with an anti-angiogenic agent and EGFR-TKI may be a potential option for extending survival outcomes in patients with the exon 21 (L858R) mutation. However, current clinical practice does not include detection of EGFR mutation subtypes; therefore, these patients may not be receiving treatments that provide the most beneficial outcomes. Patients need to be tested and informed about their mutation subtype and the available treatment options. This will help physicians make informed choices when personalising treatment strategy and improve clinical outcomes.”
Also available: Expanding Possibilities in EGFR-Mutated Advanced NSCLC: Interviews with Four Key Opinion Leaders
References
- Harrison PT et al. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167-79.
- Tamirat MZ et al. Structural characterization of EGFR exon 19 deletion mutation using molecular dynamics simulation. PLoS One. 2019;14(9):e0222814.
- Yun C-H et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11(3):217-27.
- Roskoski R Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395-411.
- Gilmer TM et al. Impact of common epidermal growth factor receptor and HER2 variants on receptor activity and inhibition by lapatinib. Cancer Res. 2008;68(2):571-9.
- Carey KD et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66(16):8163-71.
- Kobayashi Y et al. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179-86.
- Tsukada Y et al. An in vivo model allowing continuous observation of human vascular formation in e same animal over time. Sci Rep. 2021;11(1):745-53.
- Chatterjee S et al. Transient antiangiogenic treatment improves delivery of cytotoxic compounds and therapeutic outcome in lung cancer. Cancer Res. 2014;74(10):2816-24.
- Suda K et al. Clinical impacts of EGFR mutation status: analysis of 5,780 surgically resected lung cancer cases. Ann Thorac Surg. 2021;111(1):269-76.
- Nakagawa K et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655-69.
- Lee VHF et al. Differences Between the East and the West in managing advanced-stage non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2020;32(1):e1–e9.
- Soria J-C et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-25.
- Park K et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomized controlled trial. Lancet Oncol 2016;17(5):577-89.Mitsudomi T et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJT0G3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11(2):121-8.
- Rosell R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-46.
- Wu Y-L et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454-66.
- Wu Y-L et al. Efficacy according to blind independent central review: post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer. 2017;104(2017):119-25.
- Maemondo M et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-8.
- Wu Y-L et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-9.
- Zhou C et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-42.
- Sequist LV et al. Phase III Study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334.
- Wu Y-L et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222.
- Joshi A et al. Efficacy of second-line pemetrexed-carboplatin in EGFR-activating mutation-positive NSCLC: does exon 19 deletion differ from exon 21 mutation? Chemother Res Pract. 2017;2017:8196434.
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871-82.
- Goel S et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071-121.
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417-27.
- Stylianopoulos T et al. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer. 2018;4(4):292-319.
- Lee CK et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst. 2017;109:djw279.
- Saito H et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625-35.
- Seto T et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomized, multicenter, phase 2 study. Lancet Oncol. 2014;15(11):1236-44.
- Zhou Q et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(9):1279-1291.