Abstract
Historically, clinical trials in cancer medicine are, unfortunately, often poorly representative of the diverse populations who ultimately receive the intervention in real-world settings. This discrepancy could relate to age, extent of comorbidity, ethnicity, socioeconomic status (SES), and/or disability. This is particularly important, as medication efficacy and/or toxicity are known to be influenced by such variables. Many cancers also disproportionately affect individuals in underserved communities. If a highly selected cohort of individuals are recruited to a trial, theoretically, the findings should only be translated to equivalent cohorts in the community. Therefore, the more representative a trial cohort is of the target population, the more generalisable and applicable findings will be. If we aim to lessen disparities and improve equity, clinical trials must strive to become more inclusive, improving our knowledge of disease in these underserved groups, and therefore improving the care we provide to them in wider clinical practice.
This review summarises the current European perspective on this topical issue, suggesting potential strategies to proactively improve inclusivity and diversity in cancer trials, by encouraging enthusiastic collaboration between the pharmaceutical industry, healthcare authorities, study sponsors, research networks, and clinicians.
![]()
Corrigendum: Editor’s Pick: Improving Inclusivity, Equity, and Diversity in Oncology Clinical Trials: A European Perspective
Speakers: Benjamin Langley, Sophie Talas, Karim Hussien El-Shakankery, Caroline Michie
Original citation: EMJ Oncol. 2023;11[1]:68-80. DOI/10.33590/emjoncol/10303428. https://doi.org/10.33590/emjoncol/10303428.
Date correction published: 08.01.23
In the article by Michie et al. in EMJ Oncology 11.1 (pages 68-80), the following sentence has been changed from “For example, in one of the most recent practice-changing studies in TNBC, ethnicity data is not reported” to “For example, in one of the most recent practice-changing studies in TNBC, ethnicity data were not reported until recently, where <5% were Black or African American,” based on reanalysis of the literature. This has now been corrected in all versions.
The authors apologise for any inconvenience caused.
![]()
Key points
1. Clinical trials in cancer medicine have been criticised as, historically, trial populations have often not truly reflected real-world patient populations, with a significant number of underrepresented groups with respect to, for example, age, ethnicity, disability, socioeconomic status, or patients with sociolinguistic barriers. This limits the generalisability of the data, and creates an inequality for patients who could benefit from inclusion in clinical research.2. This review summarises the current European perspective on this important and topical issue, suggesting potential strategies to improve inclusivity and diversity in cancer trials, drawing on evidence to date from other countries.
3. To improve diversity in clinical trials, a proactive approach, with collaboration between the pharmaceutical industry, healthcare authorities, study sponsors, research networks, and clinicians, will be required. Only when these inequalities have been properly addressed, can we say that clinical trials are truly representative of the populations they serve.
BACKGROUND
Although clinical trials form the backbone of evidence-based medicine, cohorts participating in clinical trials are often poorly representative of the diverse populations who ultimately receive the intervention in real-world settings. This discrepancy may relate to age, extent of comorbidity, ethnicity, socioeconomic status (SES), and/or disability.1 This is particularly important, considering the medical literature tells us that medication efficacy and/or toxicity can be influenced by age, sex, ethnicity, and other variables.2 We also know that many cancers disproportionately affect individuals in underserved communities.3,4 If a highly selected cohort of individuals are recruited to a trial, theoretically, the findings should only be translated to equivalent cohorts in the community. Therefore, the more representative a trial cohort is of the target population, the more generalisable and applicable findings will be.5
If we aim to lessen disparities and improve equity, clinical trials must strive to become more inclusive, improving our knowledge of disease in these underserved groups, and therefore improving the care we provide to them.5 Prior to discussing this further, the authors define diversity, inclusivity, and equity below in Table 1.1,6

Table 1: Key terminology and definitions.
In 2022, the U.S. Food and Drug Administration (FDA) issued guidance on improving trial racial inclusivity, including a requirement for all trial sponsors in the USA to formally submit a ‘Race and Ethnicity Diversity Plan’. The plan should highlight any existing evidence as to whether efficacy of the trial intervention/agent is influenced by race and/or ethnicity, alongside listing ‘goals and plans for enrolment of underrepresented racial and ethnic participants’.7 Supporting this, the United States Congress’ end-of-year budget allocated funding to develop interventions that improve trial diversity.8 Presently, no such requirements exist for other underrepresented groups.
This review explores the challenges that underserved groups face regarding clinical trial involvement. With the USA taking proactive steps to counteract such issues, the authors consider what Europe and its leading organisations are doing to improve trial inclusivity, alongside discussing various innovative solutions aimed to improve recruitment and the representation of underrepresented parties. In writing this review, for each key inequality challenge, a separate search was undertaken using the individual challenge as a search term, together with “clinical trials,” in order to select appropriate literature. The authors focus here on bringing together a European perspective.
CHALLENGES
Ethnicity
An ethnic group is defined as a large collection of ‘people classed according to common racial, national, tribal, religious, linguistic, or cultural origin or background’, while an ethnic minority group describes those ‘living in a country where most people are from a different ethnic group’. In contrast, race is defined as a group in which people belong, classified by physical characteristics one perceives them to share; although the definition shares similarities with ethnicity/ethnic groups, the terms should not be used interchangeably.9-11
In 2022, the National Institute for Health and Care Research (NIHR) reported that only 60% of 148 UK randomised controlled trials (RCT) provided data on participant ethnicity.12 Furthermore, in trials that do report ethnicity, underrepresentation is often apparent. For example, Smith et al.13 analysed data from 943 RCTs crucial to medication authorisation by the European Commission (EC), showing that Black and Asian populations were underrepresented in almost 50% of trials. The review highlights the abundance of literature discussing trial ethnic diversity stemming from the USA, relative to European counterparts; this is perhaps driven by a combination of historical and attitudinal differences.13 Despite knowing that ethnic minorities in Europe also face disparities in cancer care, one must consider whether European research is comparably as committed to prioritising equity for these groups.14,15
Aimed at enhancing trial ethnic diversity, Schwartz et al.16 state three key goals: building trust, promoting equity, and increasing biomedical knowledge. Described in American literature, distrust of the medical profession draws on historical abuses and modern-day inequalities.17,18 However, European literature on this subject is lacking, so whether the reasons underpinning American distrust translate to this population is difficult to say. Secondly, equity recognises that potential benefits of trial participation should be made accessible to all, regardless of background and characteristics. This benefit comes not only from the trial intervention, but also from participation in general; inequity limits access to groups that may need it most.19-22 Such benefits include dedicated support by a trials team, enhancing continuity of care, and increased (and often quicker) access to investigations, blood tests, and appointments with a healthcare professional.19,20,23 Finally, biomedical knowledge is gained through diversity, because cohorts highly representative of the diverse target population increase the generalisability of results. Pharmaco-ethnicity considers ethnicity-dependent variations in treatment response and toxicity,24 and though individual trials are usually underpowered to robustly identify these differences, it emphasises why inclusivity makes for more applicable data.16
Indeed, achieving equity may not mean proportional representation with regard to the general population, but representation weighted to reflect that some cancers affect certain ethnicities to a greater degree. For example, mortality incidence in African American patients with triple negative breast cancer (TNBC) is almost twice that of White counterparts.25,26 Nevertheless, social factors may play a significant role in TNBC survival in this group, which may also form barriers to trial participation, widening inequity.26 However, the pressing need to improve care for this subgroup is not reflected in clinical trial recruitment. For example, in one of the most recent practice-changing studies in TNBC, ethnicity data were not reported until recently, where <5% were Black or African American.27,28
We must also recognise that ethnicity intersects with socioeconomic, educational, language, and geographical factors, all of which may contribute. The term ‘ethnic minority’ refers not to a homogenous population, but describes diverse peoples with different cultures, history, and genetics. Moreover, Europe is a diverse continent, with variation between countries not only in ethnic and linguistic makeup, but in healthcare access, resources, governing bodies, historical tensions, politics, migration, and attitudes towards equality. Therefore, inclusion strategies must be both multifaceted, and regionally tailored.
Language and Health Literacy
Language is a well-recognised barrier to trial inclusion.29-35 For an individual to provide capacitous consent, they must be able to understand, retain, weigh up, and communicate their decision.36 The Centers for Disease Control and Prevention (CDC) defines health literacy as the ability of individuals to “find, understand, and use information and services to inform health-related decisions and actions for themselves and others.”37
Alongside verbal explanations, trials also involve significant volumes of written information and paperwork, including written consent. It may not always be immediately evident that a language barrier exists. For example, someone with good conversational English may have difficulties with written language, and/or be unfamiliar with medical jargon. National Health Service (NHS) England’s Increasing Diversity In Research Participation guide recognises further language-related barriers, like poor health literacy, culturally inappropriate explanations, use of acronyms, and poor access to quality translation.29 A 2020 study objectively assessing the readability of patient information documents from 154 different UK clinical trials found that not one met the recommended mean reading age of <12 years, recommended by the American Medical Association (AMA).38 Consideration should also be applied to communication needs in populations prone to language barriers, including visually/hearing impaired, cognitively impaired, learning disability groups, and groups for whom the trial participant’s first language differs from the primary language used in the country that the trial is based.30,39-41
Sociolinguistic Disabilities
Communication of trial information can be equally difficult for sociolinguistic minorities, such as those with impaired hearing or vision. Without adequate accessibility and support for these patients, lower involvement in clinical trials is inevitable. Deaf patients are particularly prone to lower levels of health literacy, and may also have unique reservations with regard to research participation, such as fears of confidentiality breaches via translators likely to be within the same social circles as themselves.41
Rural Communities
Several studies, primarily conducted in the USA, have demonstrated that people from rural communities may have a shorter life expectancy,42 present with more advanced cancers,43,44 and are less likely to participate in clinical trials.42,45,46 Possible reasons for this are numerous, including lower education and SES in rurality,37 patient perception that healthcare is less accessible,39 fear of associated costs,41 and poor trial recruitment effort rurally.47 To some degree, many of these likely translate to corresponding European populations.
Individuals living in remote areas are considered an underserved group by the NIHR Innovations in Clinical Trial Design and Delivery for the Under-served (INCLUDE) project.34 Regarding healthcare distributions, Europe has a distinct rural-urban divide, concentrating workforces in urban populations.35 A 2020 UK-based YouGov poll of clinical trials participants found that those in urban regions travelled 10–20 miles on average for cancer care, while rural participants travelled an average of 20–50 miles.48 Additionally, despite modern solutions to increase digital presence, and minimise face-to-face contact for participants, rural communities may still find themselves digitally isolated.30
Socioeconomic Status
Cancer incidence is known to vary according to SES, with cancers such as cervical and lung more common in those of low SES living in income-deprived areas.49 Despite those from deprived areas representing a significant proportion of the general cancer population, most individuals enrolling in clinical trials tend to be affluent, limiting generalisability of the data to real-world settings.8,50,51 Created by the UK government, the Index of Multiple Deprivation (IMD) score is a measure of socioeconomic deprivation, summarising how deprived individuals living within a geographical area are; it is often considered deeply related to socio-economic status.52,53 Supporting the above, Noor et al.54 observed that patients with an IMD score of 5 (most deprived quintile) were significantly less likely to be referred for early Phase (Phase I) trials in an England-based cancer centre (odds ratio: 0.53; 95% confidence interval: 0.38–0.74).
Potential barriers have been postulated that may prevent deprived individuals from accessing trials, including lower health-seeking behaviours, distrust, lack of awareness/understanding, and language/cultural barriers.55-58 Furthermore, in those showing interest, associated costs may also limit engagement. These include expenses for more frequent travel, commonly to trial centres further away than their local hospital, secondary to additional appointments, and more frequent imaging.59,60 Time away from their employment and/or other caring responsibilities may also deter individuals from lower SES more than those who are affluent. Furthermore, poor retention in clinical trials has commonly been attributed to financial, time, or other practical reasons.61
With research highlighting a clear relationship between SES and physical health, those of lower SES may also be disproportionately excluded from trials via eligibility criteria that include various comorbidities and lower performance statuses. Several factors may result in worse physical health in those of lower SES, including housing conditions, nutritional status, income, and the likelihood to undertake manual labour work.62,63
Elderly Populations
Considering older individuals are more likely to be frail and/or comorbid, alongside being more susceptible to treatment toxicities, it is perhaps easier to understand the reduced representation of elderly patients in clinical trials involving potentially harmful interventions. Additionally, they may have different perspectives on quality versus quantity of life. However, despite these factors undoubtedly influencing patient eligibility, evidence suggests older patients are no less willing to participate in research. As 42% of the cancer population are >70 years, their participation is valuable. Despite this, only 10% of this cohort are involved in National Cancer Institute (NCI)-sponsored clinical trials.64,65 Therefore, one must consider if unnecessary barriers are preventing involvement.
In particular, we understand that there are notable biological differences in the handling of medications by the elderly. These include changes in gastric acid production and gut transit time (influencing absorption and peak drug concentration), declining serum albumin, impaired hepatic and renal drug clearance, and differences in body composition.66 These may serve to alter the therapeutic benefits or possible side effects of medications, and the overall effect of a particular drug in this population will only be truly established when opportunities are provided to test them. Indeed, any appropriate dose adjustments could be best defined prior to a treatment going to market.
Alongside drug metabolism concerns, a 2021 systematic review identified several other barriers at both care provider and patient levels.64 These included strict eligibility criteria, choice of language in consenting, reluctance from healthcare professionals secondary to fears of toxicity or comorbidities, poor awareness of available trials, and even general concerns solely around the patient’s age. Finally, patients themselves may lack comprehensive understanding of what trials entail, and/or may worry about logistical implications, such as reliance on family members for travel, and time commitments.
There has, however, been some progress, with the European Organisation for the Research and Treatment of Cancer (EORTC) Elderly Task Force initiating an Older Adult Council thinktank to ‘actively promote clinical and translational research in older adults over 70’. Several of their tumour subgroups have collaborated with this taskforce to initiate a number of trials dedicated to elderly populations.67 Furthermore, geriatric oncology is also now a specialist field in its own right, promoted and supported by the International Society of Geriatric Oncology (Société Internationale d’Oncologie Gériatrique [SIOG] in French). This was set up in 2000 in Europe as a multidisciplinary network, and presently boasts members from >80 countries worldwide. Research and education are two of its main strategic priorities.68
POSSIBLE SOLUTIONS
Reasons for underrepresentation of the above groups are both numerous and broad. Though some innovative ideas may benefit more than one population, no one-size-fits-all solution exists; each group should be considered in turn to optimise outcomes.
The INCLUDE Framework
Several European groups have been working to improve inclusivity of underserved groups, including the NIHR and their INCLUDE framework, which considers several under-served populations, including those listed in Table 2. The guidance encourages research teams to carefully consider their target/study population, specifically highlighting relevant underserved groups, and any potential barriers to participation. Through helpful definitions, examples, and questions, the framework prompts researchers to reflect on the inclusivity of their work.34 In considering inclusion and exclusion criteria, research teams are strongly encouraged to justify why such criteria are merited if they are likely to prevent underserved groups from being eligible to participate.34
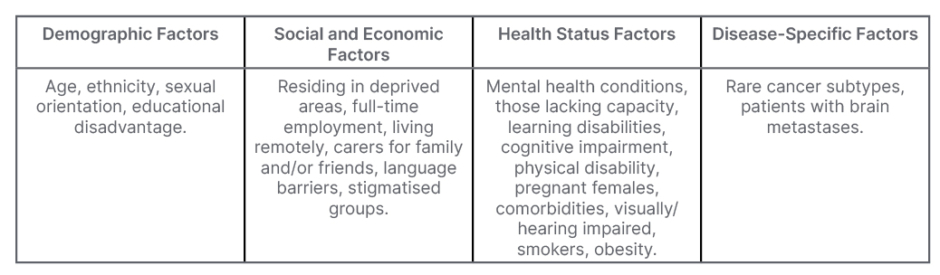
Table 2: Underserved groups highlighted within the National Institute for Health and Care Research (NIHR) INCLUDE project.
Possible Solutions: Ethnicity
To generate solutions, one must first consider the many postulated barriers to participation faced by ethnic minorities. These include distrust in medical communities;17,29-33,69 lacking knowledge around trials;30 financial worries;29,30,32,34 associated logistical issues, such as transport, or care responsibilities;30,31 cultural barriers, including stigmas about disease;29-34 language barriers;29-35 and excessive exclusion criteria.30-34 Multiple studies suggest that ethnic minorities are no less willing to participate than their counterparts, and so the discrepancy cannot be dismissed as purely attitudinal.70,71
Of the numerous proposed and tested strategies, the most notable include: cultural competency training, where trial staff are educated in respecting, understanding, and communicating appropriately with patients from different cultures;29,30,72,73 video education interventions that provide simple, culturally-sensitive explanations of trials;34,73 community-based approaches, e.g., partnership with community organisations and leaders, alongside involving them in study design/implementation;30-32 and use of trusted or respected medical professionals.32 Non-discriminatory and carefully selected exclusion criteria also consider that certain comorbidities, for example, are more prevalent amongst particular minority groups, and may not truly need to be excluded.30,33,34 However, strategies must avoid becoming coercive, or paint an unbalanced view of trial involvement.
Looking forward, the INCLUDE project has produced a catalogue of available multimedia resources and an ethnicity framework,74 amongst other resources, which challenge teams to evaluate their trial design. Similarly, the UK Engineering and Physical Science Research Council (EPSRC) has produced an Equality, Diversity, and Inclusivity (EDI) strategy, encompassing a variety of themes, from good recruitment practices to accessible environments, as well as diversity of study recruitment.75 This effectively demonstrates that EDI should permeate all aspects of research, and that inclusive trials should be the fruit of inclusive organisations. Furthermore, ‘Increasing Diversity in Research Participation’, a good practice guide from NHS England, proposes and categorises practical recommendations into pre-research planning, involvement, and respect during research, and post-research feedback and improvement.29 As one of its key targets within the 2021-22 Clinical Research Delivery Implementation plan, the UK government has also published several recommendations, with the intention of working with the NIHR to make research ‘more diverse and more relevant to the whole UK’.76
Outside of government-led organisations, Egality are a startup agency with the goal of improving clinical trial diversity by facilitating the engagement of researchers with diverse communities.77 Through these relationships and practical experience, they have generated recommendations targeted at the national, organisational, and individual level, to encourage the first steps towards improvement.77 Meanwhile, the European Medicines Agency (EMA) stress the importance of diversity in several of their International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines, emphasising representative participant selection.78,79 The Medicines and Healthcare products Regulatory Agency (MHRA) are also set to release a new framework that contains guidance on improving diversity in trials.80
However, despite the above concerted efforts, there remains no requirement for trial publications to report ethnicity data (unlike examples in the USA),81 and no standardised way of recording this.12 In the meantime, it remains to be seen whether European nations value inclusive trials, and are making meaningful steps forward.
Possible Solutions: Language and Sociolinguistic Minorities
Considering language and its associated barriers, multiple solutions exist. Using bilingual staff has been shown to be effective, as has access to professional interpreters.30 Written material available in the participant’s preferred language can be helpful,30,31 but straightforward translation of documents may not give much benefit, and a more tailored approach is needed;32,82 these include pitching writing at an appropriate literacy and cultural level, creating simpler and more concise documents, and making use of multimedia materials.29-31,72,82 Non-written patient education materials, such as videos, pictographs, and audiotapes, have been shown to increase patient understanding,83 and trial information videos with sign language would further facilitate participation of deaf patients.34,84 It is also important to recognise not only the huge volumes of languages widely spoken in diverse European countries, but also the nuances of different languages. One example of this is Gujarati, which is predominantly a spoken language, making written translations largely redundant.32 Additionally, the language does not have a word for cancer, so much care has to be taken to communicate concepts effectively.32 This highlights the importance of cultural competency alongside language tools, seeking to ensure communication is not only effective, but considerate and informed.72
Possible Solutions: Rural
Proposed strategies for reducing inequalities for rural patients in clinical trials include employing technology for virtual appointments;85 reimbursing expenses;4,46,85 providing transport;86,87 and using satellite sites for clinics, appointments, scans, and/or treatments.88 Improving physician education around the challenges for rural populations will also be imperative.45,46,87 One organisation actively addressing the urban-rural divide is the International Institute for Rural Health (LIIRH), based in Lincoln, UK.89 In particular, they hope to use the European Code of Cancer Practice, which summarises the pillars of a quality cancer service, to evaluate rural cancer care.90 The current paucity of research into rural population involvement in European clinical trials means these communities are likely to remain underserved.
Possible Solutions: Socioeconomic Status
Despite disparity in oncology trial participation having always been present, only now is there a slowly growing body of research attempting to address it. The NIHR-INCLUDE guidance contains a ‘Socioeconomic Disadvantage Framework’,34,91 which specifically seeks to increase participation of poorer patient groups. The framework also encourages research teams to ensure trial outcomes are relevant to the broader population, and not just the typically over-represented affluent trial participants. Four key questions are proposed to encourage trial organisers to optimise inclusion of those of lower SES:
1. Are people from different socioeconomic backgrounds likely to respond to the intervention in different ways?
2. Will my trial intervention and/or comparator make it harder for people from different socioeconomic backgrounds to take part in the trial?
3. Will the way I have planned and designed my trial make it harder for people from different socioeconomic backgrounds to take part in the trial?
4. What factors might affect the reporting and dissemination of trial results?
Patients of lower SES are more vulnerable to the financial stressors of trial participation, including travel and childcare costs. To improve recruitment and retention, trial sponsors must consider compensating for such expenses, and addressing concerns of potential trial participants.
Possible Solutions: Elderly Patients
Reassuringly, more seems to have been actioned at a legislative level with regard to elderly involvement in trials.
The guideline ICH E7, initially published in 1994, laid out the fundamental principle that “drugs should be studied in all age groups, including the elderly, for which they will have significant utility.”92 Their guidance made specific requirements for adequate representation of elderly patients, with at least 100 older patients included in trials where the disease is not unique to the elderly. They clearly state that upper age limits should be avoided, as should excluding patients with comorbidities, where possible. The guideline also highlighted the importance of pharmacokinetic and pharmacodynamic studies in this population, alongside research into novel drug-drug interactions. A decade later, the World Health Organization (WHO) encouraged the development of laws and guidelines that would obligate the inclusion of the elderly in clinical trials, stating that these patients were ‘unjustifiably excluded’.93 They advocated for tailored formulations of drugs for the elderly, to maximise adherence, and recommended thorough monitoring for adverse reactions.
In 2006, the EMA reviewed 10 dossiers of recently approved medications to assess their compliance with ICH E7, which, on the whole, showed good compliance.94 They also made recommendations for how they believed ICH E7 could be improved upon, such as necessitating the involvement of both the elderly and very elderly participants as discreet subgroups in the clinical testing of medications.
While guidelines have laid groundwork for better inclusion of the elderly, barriers preventing optimal patient involvement still remain. One proposed solution is ‘geriatrising’ trial design;64 this includes trying to use standardised measures of frailty, and choosing appropriate endpoints, such as active life expectancy, as well as specifically designing studies that investigate dosing, tolerability, benefits, and toxicities in the elderly.95 Indeed, these measures will require widespread support alongside commitment of dedicated funds and resources for success. Easing logistical barriers through telehealth appointments, or assisting with travel and/or lodging, may also encourage enrolment.64,96 Furthermore, improving communication and providing information tailored to elderly patients is another important step in aiding accessibility and understanding. Finally, psychosocial barriers, such as physician bias, must be tackled; in part, this may be addressed through availability of high-quality data and literature, aiding clinicians to make evidence-based decisions.96
PHARMA PERSPECTIVE
Data from the EMA suggest that of approximately 2,800 clinical trials authorised each year for human medicines, 60% of these are commercially funded. Hence, the pharmaceutical industry must show active engagement in processes to improve diversity, and enact meaningful change.97 Since the COVID-19 pandemic, improving diversity has become a hot topic of discussion. Literature stemming from several companies highlights trial diversity as an area of unmet need.98-102
Companies are already starting to implement many of the solutions discussed above; notable examples include community engagement and advocacy groups,98,99,102 diversity-specific staff training, increased recruitment of female and minority ethnic trial investigators,99 increased ethnic minority representation within pharmaceutical organisations alongside gender parity,98 and dedicated/ringfenced funding to ensure equity and diversity commitments are upheld within organisations and trials.98 Other companies are also setting their own in-house targets, aiming for >75% of trials to have an outlined participant demographic plan, specifically and appropriately aligned with the epidemiology of the disease being studied; this was achieved in 100% of their Phase III studies in 2022.102
Aiming to build trust, work is also underway to improve access to patient-facing materials, such as videos to explain trials in multiple languages, and patient testimonies of trial involvement, especially from participants of underserved groups (unpublished correspondence). It should be highlighted, however, that these resources should be treated the same as any patient information correspondence, and therefore will be subject to the same regulatory and ethical restrictions, incurring cost and time. It is imperative that any digital communications are used as explanatory aids, and not as an ‘advert’. Furthermore, confidentiality, and the ability to easily share digital resources further than their intended population, is also a risk that must be balanced (unpublished correspondence).
FUTURE DIRECTIONS AND STRATEGIES
Though many solutions show great potential, ensuring these are implemented in practice remains challenging. Although progress is being made, more is required. Previous research, primarily from the USA and the FDA, has demonstrated that legislation and recommendations from official public bodies, with associated accountability for actions, does translate to effective outcomes.8 At present, no such legal requirements have been proposed by European organisations.
In a similar vein, the Fair Inclusion Score (FIS) for clinical trial diversity has been developed, generating an index/score based on trial participant sex, age, and ethnicity.8,103 The score, intended to be publicly visible alongside published research, considers transparency of demographic data in published trials, alongside demographic representations in those that do present this data, compared to official demographics of the relevant target population. The score can be utilised on any published RCT, providing a diversity ‘quality measure’ that can be considered overall, or split into female, age, and ethnicity subgroup scores. Scores may then be compared to an acceptable ‘benchmark’, or directly compared with other trials and/or organisations, highlighting the efforts that organisations are, or are not, making to ensure their clinical trials are generalisable. As part of its development, retrospective application of the FIS on 69 RCTs (leading to FDA approval of 59 novel therapeutics between 2012–2017) showed that trials often displayed good transparency (mostly age and gender, with fewer studies noting ethnicities), but poor demographic representation, consistent with previous findings. Although the score highlights and considers diversity of trial populations after publication, this is performed retrospectively, and does not play a role in improving inclusivity prospectively.8,103
Similarly, organisations in the USA, such as the National Academies of Sciences, Engineering, and Medicine, are calling on journal editors and publishers to consider trial representation and inclusivity in all submissions.1 However, changes must be considered far earlier than this, hence the proposal of a Diversity, Equity and Inclusion Clinical Trial Life Cycle (DEICTLC), produced by Versavel et al.1 Somewhat similar to the NIHR-INCLUDE project, the DEICTLC outlines each stage of trials from planning to close, with a useful checklist of actions that can be undertaken at each stage to maximise inclusion of underserved groups. Though the DEICTLC is in its infancy, and more evidence around its use in real-world practice is required, it shows great potential as an aid for study sponsors. In the UK specifically, organisations such as MedCity have themselves released guidance on trial inclusivity, with recommendations largely echoing those previously noted above. They also call for official UK organisations such as the Association of the British Pharmaceutical Industry (ABPI) and the Prescription Medicines Code of Practice Authority (PMCPA) to formulate code of practice regulations for trial sponsors, formalising guidance which will enhance community outreach and engagement.104
CONCLUSION
Though steps are being made towards improving clinical trial EDI, there remains significant room for improvement, particularly in Europe. Using a combination of general and targeted approaches, academic and commercial organisations must strive to implement innovative solutions at all stages of clinical trials, to recognise and act on the disparities underserved groups experience. Only when these have been addressed, can we say our clinical trials are truly representative of the populations they serve.







