Abstract
Extracranial germ cell tumours (GCT) are derived from dysregulated, unipotent to totipotent, primordial germ cells and can arise from heterogeneous sites and occur across a broad age range of patients. Although healthcare professionals in the paediatric and adult medical fields collaborate closely, discrepancies in the staging system and risk-assignment used still exist. Treatment outcomes are worst in adolescent patient groups. Surgical principles have been established for treatment at initial diagnosis and during salvage therapy, as well as for the most difficult circumstances, termed desperation surgery. The development of cisplatin-containing chemotherapy marked the 1st success in GCT treatment, representing one of the major advances in the last 50 years of modern oncology. Nowadays, first-line three-drug chemotherapy regimens use cisplatin, etoposide, and either bleomycin or ifosfamide. Paediatric chemotherapy regimens typically reduce the use of bleomycin or replace cisplatin with carboplatin to decrease the levels of toxic agents in developing children. New targeted chemo-agents have been explored as potential options for refractory and relapsed GCT, as well as non-GCT malignant transformation. Here, the chemotherapy regimens currently used by paediatric and adult oncologists are described. The recent progress in targeted chemo-agents that are being used in the clinic is also discussed. Hopefully, through appropriate delivery of targeted chemo-agents, combined with well-established surgical procedures, the best outcomes of GCT for every age population can be achieved at initial diagnosis and for relapsed/refractory GCT and non-GCT transformation.
INTRODUCTION
Extracranial germ cell tumours (GCT) can arise at heterogeneous sites, can occur across a broad age range of patients, and are treated by a variety of specialists, including paediatric oncologists, medical oncologists, urologists, or gynaecologic oncologists.1 The common sites of extracranial GCT presentations include the gonads (testes or ovaries) and extragonadal sites (mediastinum, retroperitoneum, and sacrococcyx).2 Due to the totipotent nature of primordial germ cells, GCT comprise a broad range of histologic subtypes, including germinomas (testicular seminomas and ovarian dysgerminomas), embryonal carcinomas, teratomas, yolk-sac tumours (formerly known as endodermal sinus tumours), and choriocarcinomas.2 Tumours that contain various malignant histologies are termed mixed malignant GCT.2 The outcome for adolescents with GCT is worse than for young children and adults, as reported by an institutional database and validated within the Malignant Germ Cell Tumours International Collaborative database.2,3 As stringent evaluations, disciplined surgeries, and appropriate chemotherapies are important for successful treatment of GCT, the under-representation of adolescents in either paediatric or adult clinical trials might have compounded the poorer outcomes in this particular age group.2 Thus, collaborations between paediatric and adult oncologists, such as in the AO31102 study (the TIGER trial;4 recruiting patients >14 years old), are welcomed. Current strategies for GCT treatment are comprehensively risk-based and incorporate multi-disciplinary surgical and/or chemotherapy protocols, mainly derived from the experiences of adult testicular GCT management.5,6 However, there are significant differences in staging and risk assignment among different collaborative groups, especially between paediatric and adult oncology professionals.1,2 Such differences can particularly affect the transitional age population, i.e. adolescents and young adults, who may receive heterogeneous GCT treatment strategies under paediatric or adult staging and risk stratification.
Surgical principles have been defined for GCT treatment at diagnosis, for resection of residual disease post-chemotherapy, as well as for relapsed or refractory disease as salvage or desperation surgery.5,6 Chemotherapy has been known to improve long-term disease-free survival for GCT patients by 5–10% in those previously treated with actinomycin-D–based regimens, and by 50–60% due to the landmark success of cisplatin-containing regimens since the 1970s.7 Given the toxicities associated with cisplatin-based regimens, including adverse effects on reproductive health and hearing impairment, especially in children and young adults, these regimens have evolved and diversified, particularly in the first-line treatment setting, without compromising the results.1,2 The best salvage chemotherapy regimens have yet to be defined for relapsed/refractory GCT. In addition, current chemotherapy regimens are not effective in GCT-associated synchronous haematological malignancies, non-haematological solid malignancies, or late non-GCT malignant transformation.8,9 However, new targeted chemo-agents, with or without immune modulation and stem cell transplantation, may improve the outcomes of these conditions. We review the current use of chemotherapy for extracranial GCT in paediatric, adolescent, and young adult patients, and highlight the differences in the approaches used by various collaborative groups.
UPFRONT CHEMOTHERAPY
Risk Assignment Before First-Line Chemotherapy
Suitable staging of newly diagnosed GCT is essential before initiating chemotherapy to ensure appropriate risk assignment. Well-known and comprehensive staging systems for GCT include the American Joint Committee on Cancer (AJCC) TNM system for testicular GCT, the International Federation of Gynecology and Obstetrics (FIGO) system for ovarian GCT, and the Children’s Oncology Group’s (COG) staging system for testicular/ovarian/extragonadal GCT.1,10,11 These different staging systems may imply different chemotherapy strategies; for example, whether chemotherapy is indicated in subsets of immature teratomas differs between gynaecological and paediatric oncologists.12,13 In this context, a recent pooled analysis of patients with ovarian immature teratomas showed similar outcomes between 98 paediatric patients (treated mostly with surgery alone) and 81 adult patients (treated with both surgery and chemotherapy), and recurrences were limited almost exclusively to patients with Grade 3, Stage III or IV tumours.14 Table 1 illustrates two major risk assignment systems that were developed according to data on paediatric (the Malignant Germ Cell Tumors International Collaborative) and adult (the International Germ Cell Cancer Collaborative Group) GCT populations. Both systems trichotomously categorise GCT patients into low-risk/good-prognosis, standard-risk/intermediate-prognosis, and poor-risk/poor-prognosis groups.15,16 Both risk assignment systems take into account the primary tumour sites, histologic features, metastases, and serum tumour markers, though only the Malignant Germ Cell Tumors International Collaborative considers the influence of age, especially in the youngest population. An observation-only strategy, with active surveillance prescribed after initial surgery, has been applied to patient subsets according to different definitions in clinical Stage I/II, as well as in low-risk/good-prognosis GCT groups. Although once a standard treatment, adjuvant radiotherapy directed to the retroperitoneal lymph nodes for Stage I seminomas to reduce relapse has been largely abandoned due to the increased risk of secondary malignancies.17 Chemotherapy has been recommended for most standard-risk/intermediate-prognosis and poor-risk/poor-prognosis groups in the first-line neo-adjuvant or adjuvant setting. Incorporation of new biological agents with novel combination chemotherapies has been attempted in the context of refractory or relapsed GCT, as well as non-GCT transformation.
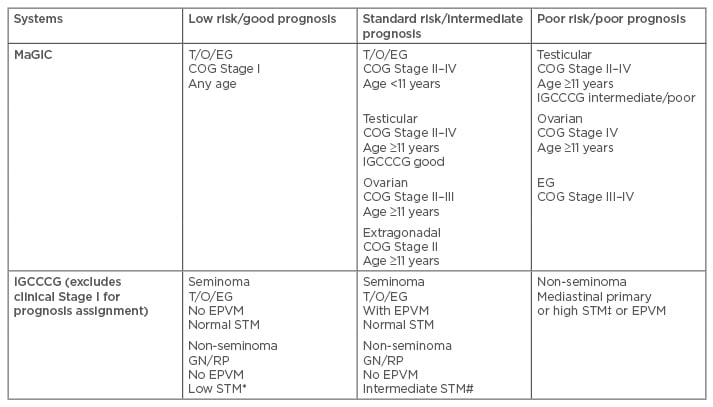
Table 1: Trichotomous categorisation of extracranial germ cell tumour by MaGIC and IGCCCG.
*Low STM: AFP <1,000 ng/mL, HCG <5,000 U/L, LDH <1.5 x ULN; # Intermediate STM: AFP 1,000– 10,000 ng/mL or HCG 5,000–50,000 U/L or LDH 1.5–10 x ULN; ‡High STM: AFP >10,000 ng/mL or HCG >50,000 U/L or LDH >10 x ULN.
AFP: alpha-fetoprotein; COG: Children’s Oncology Group; EG: extragonadal; EPVM: extrapulmonary visceral metastases; GN: gonadal; HCG: human chronic gonadotropin; IGCCCG: International Germ Cell Cancer Collaborative Group; LDH: lactate dehydrogenase; MaGIC: Malignant Germ Cell Tumours International Collaborative; RP: retroperitoneal; STM: serum tumour markers; T/O/EG: testicular/ovarian/extragonadal; ULN: upper limit of normal.
First-Line Chemotherapy Regimens
The standard BEP (bleomycin, etoposide, cisplatin) regimen, involving three weekly bleomycin treatments per 21-day cycle, remains the most popular GCT treatment.18 This standard BEP regimen, given for one to four cycles mainly in adult testicular GCT studies, has produced better outcomes in randomised trials compared to regimens substituting carboplatin for cisplatin or regimens that reduce or eliminate bleomycin.19,20 However, the paediatric PEB regimen (also bleomycin, etoposide, cisplatin), with a reduced frequency of bleomycin treatment to once every 3 weeks (i.e. once per cycle) concerning pulmonary toxicities in the developing lungs of children, has resulted in a 6-year event-free survival of 95.0% and overall survival of 95.7% in a paediatric GCT multicentre trial.21
In instances where bleomycin-induced pulmonary complications are a concern, the VIP/PEI (etoposide, ifosfamide, cisplatin) regimen is an appropriate alternative that has been shown to have non-inferior results (2-year failure-free survival of 63% versus 60% and overall survival of 74% versus 71%) compared to the standard BEP regimen in a randomised trial.22 Rates of pulmonary morbidities and mortalities after second-look surgery for mediastinal non-seminomatous GCT have been decreased using neo-adjuvant VIP compared to those using the standard BEP regimen.23
Carboplatin has been given as a first-line, adjuvant non-radiotherapy measure to reduce the relapse rate for clinical Stage I seminoma in adult patients.24 Adjuvant chemotherapy with the EP (etoposide and cisplatin) regimen has been given for Stage II non-seminomatous GCT after retroperitoneal lymph node dissection in order to prevent relapse.25 Although trials in adults have shown carboplatin to be inferior to cisplatin, the JEB (carboplatin, etoposide, bleomycin) regimen, which substitutes carboplatin (using higher doses than prescribed in the adult trials) for cisplatin, has shown 88% and 91% 5-year event-free survival and overall survival, respectively, in a multicentre childhood GCT trial, with no reports of sensorineural hearing loss.26
Attempts at incorporating other chemotherapeutic agents to the standard regimens have shown mixed results. Combining cyclophosphamide with PEB for paediatric GCT patients in the poor-risk category showed no clear improvement in event-free survival.27 A single arm Phase II trial introducing paclitaxel in the TIP (paclitaxel, ifosfamide, cisplatin) regimen, adapted for outpatient administration, has generated excellent outcomes, with impressive statistics for 3-year progression-free survival (63% and 90% for poor-prognosis and intermediate-prognosis patients, respectively) and for overall survival (87% and 100% for poor-prognosis and intermediate-prognosis patients, respectively) when given as the first-line chemotherapy for GCT patients.28 This, however, should be considered experimental until further investigation and confirmation.
Many studies, both in paediatric and adult trials, have tried acceleration or intensification of chemotherapy, as well as dose escalation after the 1st cycle, in attempts to improve the outcome of GCT. Most of these studies have not resulted in prominent improvements and were associated with unacceptable toxicities.29 One exception is that a marginal improvement in 3-year progression-free survival (59% versus 48%) was shown in a randomised trial for the dose dense arm incorporating agents, including paclitaxel, ifosfamide, and oxaliplatin, compared to the continuing standard BEP arm in subsets of patients whose tumour markers declined only slowly. The regimen, however, failed to show a significant difference in overall survival.30
SALVAGE CHEMOTHERAPY FOR RELAPSED OR REFRACTORY GERM CELL TUMOURS
Risk Assignment for Relapsed or Refractory Germ Cell Tumours
In a meta-analysis by the International Prognostic Factors Study Group, the 2-year progression-free survival of relapsed GCT after treatment with first-line cisplatin-containing chemotherapy ranged from 6% for the very-high-risk group to 75% for the very-low-risk group.31 Accordingly, a comprehensive risk assignment strategy stratified patients into five prognostic subgroups on the basis of histology, primary tumour location, response to first-line therapy, tumour marker concentrations, and sites of metastases (liver, brain, and bone).31 Clinical Stage I or good-prognosis adult patients with seminoma or non-seminoma who relapse under a surveillance strategy should be treated with 3-4 cycles of BEP or VIP, with the same recommendation under the risk assignment strategy for de novo metastases.6 Similarly, PEB chemotherapy has been applied in relapsed pre-pubertal paediatric GCT patients with excellent outcomes after surveillance strategies.32 For GCT patients who relapsed after first-line cisplatin-containing chemotherapy, it is still under debate if conventional dose chemotherapy (CDCT) is adequate, or if high-dose chemotherapy (HDCT) followed by autologous stem cell transplantation is indicated for effective salvage. An international prospective multicentre trial (TIGER study)4 comparing initial salvage HDCT with CDCT is currently ongoing to answer this question.
Conventional Dose Chemotherapy
Traditionally, most CDCT regimens for patients with metastatic GCT relapsing after first-line chemotherapy include cisplatin and ifosfamide-containing three-drug combinations. Combinations of cisplatin and ifosfamide with either etoposide (VIP/PEI) or vinblastine (VeIP) have long been used as salvage therapy for relapsed patients after first-line BEP chemotherapy.33,34 Recently, Phase II multicentre trials of paclitaxel or gemcitabine in combination with ifosfamide and cisplatin (TIP and GIP, respectively) have achieved higher response rates, as well as improved survival (Table 2).35,36 A single-institute paediatric Phase II trial using a precise delivery scheme for deep regional hyperthermia in conjunction with CDCT (PEB), has shown satisfactory results, mainly for localised relapsed sacrococcygeal or retroperitoneal GCT.37 Other new combinations, such as gemcitabine plus oxaliplatin, paclitaxel plus gemcitabine, or paclitaxel plus cisplatin plus gemcitabine, have shown responses in the most refractory subsets of GCT using observational studies (Table 2).38-40
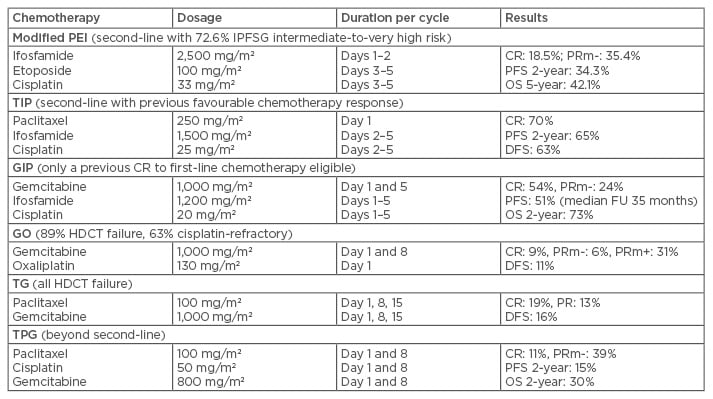
Table 2: Selected chemotherapy regimens for salvage of germ cell tumours.
CR: complete response; DFS: disease-free survival; FU: follow-up; HDCT: high-dose chemotherapy;
IPFSG: International Prognostic Factor Study Group; OS: overall survival; PFS: progression-free survival; PR: partial response; PRm-: partial response with a marker normalisation; PRm+: partial response with a marker elevation.
High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation
A retrospective analysis of 1,594 patients with relapsed/refractory metastatic GCT showed that HDCT resulted in a higher progression-free survival and overall survival than CDCT as 1st salvage treatment.41 Prospective studies comparing HDCT with CDCT are awaited to confirm this assumption. Carboplatin and etoposide remain the core components of HDCT for salvage of relapsed/ refractory GCT, but the addition of high-dose cyclophosphamide has resulted in unacceptable toxicities.42 Whether more HDCT cycles (when compared to single-cycle HDCT) will result in better GCT outcomes is still debatable. In this regard, a prospective randomised trial only revealed an improvement in 5-year survival that marginally favoured three cycles of HDCT over single-cycle HDCT (49% versus 39%). However, this finding could have been compounded by the higher doses of chemotherapies that were used in the single cycle arm, which also included cyclophosphamide, resulting in increased treatment-related mortality (14% in the single-cycle HDCT group compared to 4% in the three-cycle HDCT group).42 The TICE regimen (two cycles of paclitaxel plus ifosfamide followed by three consecutive cycles of high-dose carboplatin plus etoposide), developed by the Memorial Sloan-Kettering Cancer Center, New York City, New York, USA, has induced durable remissions, even for patients with primary mediastinal GCT. This regimen can be administered on schedule more consistently and is associated with a low risk of transplant-related mortality.43 A prospective paediatric trial has shown that complete excision of the tumour, rather than application of HDCT, is essential for the successful salvage of refractory or recurrent non-seminomatous GCT in young patients.44
Biologic Agents
The application of biologic agents in the treatment of GCT is in the early stages of investigation, with most studies being case series/case reports, thus, presenting only low-level evidence. Brentuximab vedotin is an antibody-drug conjugate consisting of the chimeric antibody SGN-30 (cAC10), which is chemically conjugated to a synthetic analogue (monomethyl auristatin E) of the anti-tubulin agent dolastatin. Brentuximab vedotin targets the CD30 antigen, the expression of which is prevalent in GCT, especially in embryonal carcinoma and mixed GCT, suggesting its potential use in the treatment of GCT. In a Phase II single-centre trial, brentuximab vedotin was given to patients with CD30-expressing GCT as salvage therapy after at least two cisplatin-based regimens, including HDCT.45 Among nine patients enrolled, there was one complete response, one partial response (>80% tumour reduction), and eight patients experienced serum tumour marker reduction after 1st cycle treatment. Five patients lost the tumour marker response after 2nd cycle treatment. It is uncertain if earlier incorporation of brentuximab vedotin will yield a better response in GCT patients.
Numerous biologic agents have been tested, mainly for chemotherapy-resistant GCT, with targets including the HER receptor family and c-KIT/ stem cell factor pathway. Anti-angiogenic agents, the multitargeted tyrosine kinase, and poly (ADP-ribose) polymerase inhibitors, among others, have been investigated.46 Until recently, no clinically meaningful activity has been shown in most trials that have investigated these targeted therapies. Nonetheless, anecdotal complete responses have been documented, including for imatinib (a small molecule inhibitor of stem cell factor receptor), in a patient with chemoresistant disseminated seminoma, and for sunitinib (a small molecule inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor, stem cell factor receptor, and colony stimulation factor receptor-1) in a patient with heavily pretreated mixed embryonal carcinoma/ yolk sac tumour.47,48 Furthermore, bevacizumab, a monoclonal anti-vascular endothelial growth factor antibody, has been incorporated into HDCT schedules and showed encouraging results in heavily pretreated and refractory GCT patients.49 Thalidomide, which has anti-angiogenic properties as well as immunomodulatory and anti-proliferative effects, has been shown to have single-agent activity in cisplatin-refractory GCT.50
Immune checkpoint inhibition may represent an effective strategy in poor-risk GCT since high expression of the cancer biomarker PD-L1 in GCT is correlated with worse progression-free survival and overall survival.51 Nivolumab treatments have induced a durable response in a platinum-refractory patient with non-seminomatous GCT.52 However, pembrolizumab treatments in a Phase II trial, with enrolment of 12 patients with metastatic non-seminomatous GCT progressed after first-line cisplatin/etoposide and/or subsequent salvage treatment including HDCT, showed no complete or partial responses, with two patients achieving stable disease at 12 and 9 weeks, respectively.53
High-Dose Chemotherapy with Post-Transplant Prophylactic or Pre-Emptive Targeted Chemotherapy
The efficacy of post-transplant maintenance therapy with targeted chemotherapy agents has been well demonstrated in subsets of haematologic malignancies, either as prophylactic or as pre-emptive strategies. Such an approach may be a useful strategy using CDCT regimens or biologic agents in relapsed/refractory GCT after HDCT. Our preliminary unpublished data indicate that treatment with a gemcitabine/oxaliplatin regimen, various targeted agents, and other chemotherapy agents resulted in 5/5 objective responses (2 partial and 3 complete), including three cases of relapsed/refractory GCT patients with high or very high International Prognostic Factors Study Group scores that maintained long-term disease-free survival (Table 3).
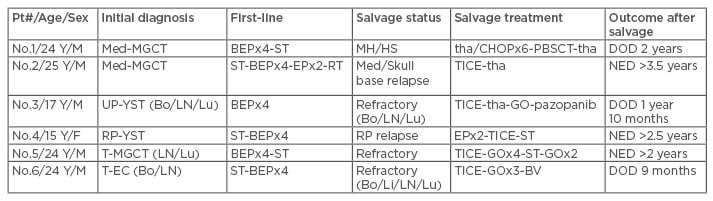
Table 3: Stem cell therapy combined with conventional dose chemotherapy and target therapy for salvage.
BEP: bleomycin etoposide cisplatin regimen; Bo/Li/LN/Lu: bone/liver/lymph node/lung metastases;
BV: brentuximab vedotin; DOD: died of disease; EP: etoposide cisplatin regimen; F: female; GO: gemcitabine oxaliplatin regimen; M: male; Med-MGCT: mediastinal malignant mixed germ cell tumour; MH/HS: malignant histiocytosis/histiocytic sarcoma; NED: no evidence of disease; Pt#: patient number; PBSCT: peripheral blood stem cell transplant; RP-YST: retroperitoneal primary yolk sac tumour; ST: surgical treatment; tha/CHOP: daily thalidomide plus cyclic cyclophosphamide adriamycin oncovin prednisolone regimen; tha: thalidomide regimen; T-EC: testicular pure embryonal carcinoma; TICE: paclitaxel ifosfamide carboplatin etoposide regimen; T-MGCT: testicular malignant mixed germ cell tumour; UP-YST: unknown primary yolk sac tumour.
CHEMOTHERAPY FOR NON-GERM CELL TUMOUR MALIGNANT TRANSFORMATION
Derived from totipotent germ cells, GCT are capable of evolving into leukaemia or variable non-GCT solid somatic malignancies. These unique scenarios can occur in GCT patients from any primary site, although it is most common in cases of non-seminomatous GCT arising from the mediastinum.54,55 Although chemotherapeutic agents for GCT, including etoposide/cisplatin, may effect leukaemogenesis, reported GCT-associated haematologic malignancies have evolved concurrently with, or shortly after, the GCT diagnosis.8,55 GCT-associated haematologic malignant cells are thought to derive from GCT and spread into the blood, bone marrow, and extramedullary sites.55 The pathology of GCT-associated haematologic malignancies is quite distinct; acute megakaryoblastic leukaemia and myelodysplastic syndrome are the most common pathologies, but malignant histiocytosis/ histiocytic sarcoma are relatively over-represented. The clinical course of GCT-associated haematologic malignancies is very aggressive, with patients either dying before treatment, not responding to antileukaemic therapy, or achieving only short remissions.8 The prognosis is extremely poor, with patients almost invariably dying within 16 months (median survival: 5 months) after diagnosis. Two large retrospective studies involving patients with therapy-related myeloid malignancies showed that allogeneic stem cell therapy resulted in a 22% 5-year and 35% 3-year overall survival.56,57 For patients who have achieved remission after antileukaemic therapy, immediate allogeneic stem cell transplantation should be considered, as case reports of such an approach showed efficacy in GCT-associated acute megakaryoblastic leukaemia and myelodysplastic syndrome.58,59 A patient with mediastinal non-seminomatous GCT-associated malignant histiocytosis/histiocytic sarcoma experienced much longer survival and a good quality of life with the use of thalidomide plus CHOP (cyclophosphamide, adriamycin, vincristine, prednisolone) chemotherapy, followed by alemtuzumab-containing reduced-intensity matched unrelated peripheral blood stem cell transplantation (Table 3).60
GCT-associated somatic malignant transformations include various carcinomas and sarcomas. Aggressive surgery is the most important treatment for curative intent.61 Among patients with unresectable GCT-associated primitive neuroectodermal tumours, the CAV-IE chemotherapy regimen (cyclophosphamide, doxorubicin, and vincristine alternating with ifosfamide plus etoposide), originally used for the Ewing’s sarcoma family of tumours, may result in better outcomes.62 However, neither GCT-type regimens nor those currently in use for specific somatic malignancies (with the exception of primitive neuroectodermal tumours) have shown efficacy in improving the outcomes of unresectable non-GCT somatic malignancies.61 Other novel strategies are needed to improve the prognosis of these particular GCT scenarios.
LATE EFFECTS OF CHEMOTHERAPY
The late effects experienced by long-term survivors after cisplatin-based regimens have been reviewed recently.2 The ototoxicity, especially in children, may result in impaired language development or hearing loss. The nephrotoxicity may cause an irreversible decrease in renal function in men with testicular GCT. The neurotoxicity, such as paraesthesia, is more often seen in adult survivors of GCT than in children. The increased prevalence of restrictive lung disease was noted in testicular GCT survivors. Also, the increased risk of cardiovascular disease and 2nd malignancy has been shown in testicular GCT survivors.
BIOLOGY OF CHEMOTHERAPY
Numerous genetic and epigenetic mechanisms have been implicated in GCT, such as gains of chromosome arm 12p, reciprocal loss of heterozygosity (RLOH) (arm level deletions with a compensatory reciprocal amplification), high intrinsic potential of cellular apoptotic propensity (so-called mitochondrial priming), missense KRAS mutations, activating KIT mutations (specific to seminomas only), as well as wild-type TP53.63 Wild-type functional TP53, RLOH, and high mitochondrial priming in GCT may cause a fundamental apoptotic propensity and contribute to the extreme sensitivity of GCT to cisplatin-containing chemotherapy regimens at initial diagnosis.
Plausible causal events that lead to the development of cisplatin-resistance in GCT have been proposed, which include continued progression of RLOH copy number events and loss of pluripotency markers (such as NANOG and POU5F1), as well as acquired mutations in the XRCC2, PIK3CA, AKT, KRAS, and NRAS genes.2,63 Targeting these events in GCT with biological therapies is yet to be explored.
FUTURE DIRECTIONS
Complex multimodal strategies are required for the successful management of GCT. It is highly advisable that GCT patients are treated in expert centres. The future goal of first-line chemotherapy for GCT is to extend the success of treatment and reduce the treatment burden so that the quality of life of GCT long-term survivors can be enhanced. Collaboration between paediatric and adult oncology professionals is essential to improve the outcome of adolescents and young adults with GCT. The development of novel chemotherapy, targeted therapy, and immune therapy, either alone or in combination, is urgently anticipated to successfully salvage relapsed and refractory GCT.








