Abstract
Ovarian cancer is the most common gynaecological malignancy and the seventh most common malignancy in women. Inherited ovarian cancer is caused by mutations in certain genes, such as BRCA1 and BRCA2, as well as many minor genes. The pathology of ovarian cancer involves damage to the cell cycle mechanism secondary to mutations in BRCA1/2 protective genes. These mutations provide a meaningful marker for screening and diagnosing hereditary ovarian cancer. Classification of ovarian cancer is based on histology, depending on which layers of the ovary are affected.
The authors conducted an electronic search using keywords and selected the included studies based on pre-established inclusion criteria. To avoid bias in the data extraction process, three reviewers extracted information independently. Risk assessment models provided by the National Comprehensive Cancer Network (NCCN) and American College of Obstetricians and Gynecologists (ACOG) are mostly used in clinical practice. The combination of serial serum cancer antigen-125 (CA-125) levels and transvaginal ultrasound is the only evidence-based screening approach available to patients at increased risk for ovarian cancer.
Strong evidence has made salpingo-oophorectomy the gold standard for risk-reducing surgery. Bilateral salpingectomy, in contrast, is restricted to clinical trials currently. The protective effects of oral contraceptives have made them suitable agents for chemoprevention. Whilst the potential benefits of aspirin and certain other drugs have been investigated, further research is required to address the gap in data for them to be used in clinical practice for the purpose of ovarian cancer prevention.
Key Points
1. Increased risk of developing breast cancer conferred by BRCA1 and BRCA2 gene mutation is the principle on which current screening test relies upon.2. Primary prevention by genetic testing has been gaining ground in recent years with the National Comprehensive Cancer Network putting forward guidelines and criteria.
3. Along with surgical management, certain drugs and substances have also been found to reduce risk by various mechanisms.
INTRODUCTION
Ovarian cancer is a serious type of cancer that affects women. It has high morbidity and mortality and is the seventh biggest cause of cancer deaths in women.1 According to Global Cancer Statistics 2022, ovarian cancer shows a 3.4% chance of occurring among almost 10 million new cancer cases, with a 4.8% mortality rate in approximately 4 million female patients with cancer.1 In India, northeastern areas like Arunachal Pradesh (specifically Papumpare District) along with Delhi have a notably high number of ovarian cancer cases.2 More developed countries, like the USA and those in the European Union, are expected to see a 42% rise in various cancers by 2050.1 Meanwhile, countries with medium development levels, like India, might face a dramatic rise of up to 100% in cancer cases during the same time frame.1 This means that the incidence of ovarian cancer could increase by approximately 10–15% in the coming decades.1
Since Mary-Claire King, University of Washington, USA, and her team identified the BRCA1 gene linked to ovarian and breast cancers, there have been huge advances in prevention and treatment.3 The BRCA2 gene was located by Michael Stratton, Wellcome Sanger Institute, Hinxton, UK, and Richard Wooster, Haddow Laboratories, Sutton Surrey, UK.4 In India, mutations in the BRCA1/2 genes account for around 25.69% of hereditary breast and ovarian cancer cases.5 Other genes like TP53, PALB2, BRIP1, and ATM also play a part, contributing to another 3.47% of hereditary breast cancers.5 Because of this high occurrence of BRCA-related ovarian cancer in India, it is important for health professionals to check risk factors and take action to detect, diagnose, and treat it effectively.
Role of BRCA Genes
The BRCA1 gene is activated upon detection of DNA damage and when there are irregularities in the cell cycle. BRCA1 is located on chromosome 17q. It is composed of 22 coding exons distributed over 100 KB of DNA. It becomes hyperphosphorylated in response to DNA damage, and it relocates to the site of replication forks marked by proliferating cell nuclear antigen (PCNA). In response to ionising radiation, BRCA1 is bound and phosphorylated by ATM kinase.6 There are proteins that regulate the action of BRCA1 during DNA repair, transcription, and cell cycle. Ionising radiation potentially destroys the interactions of proteins with the BRCA1 gene. The number of proteins that interact with BRCA1 and the arrangement of proteins on BRCA1 is highlighted in Figure 1. Similarly, BRCA2 helps to repair damaged DNA more sensitively. It is present on chromosome 13q12 and repairs chromosomal breaks and aberrant mitotic exchanges that occur during the cell cycle.6 Studies show that BRCA2 and RAD51 are fundamental for the maintenance of cell division and chromosomal structure. Proteins interacting with BRCA2 and the arrangement of proteins on BRCA2 are highlighted in Figure 1. Proteins interacting with BRCA1 and BRCA2 and the arrangement of proteins on them are discussed in Figure 1.
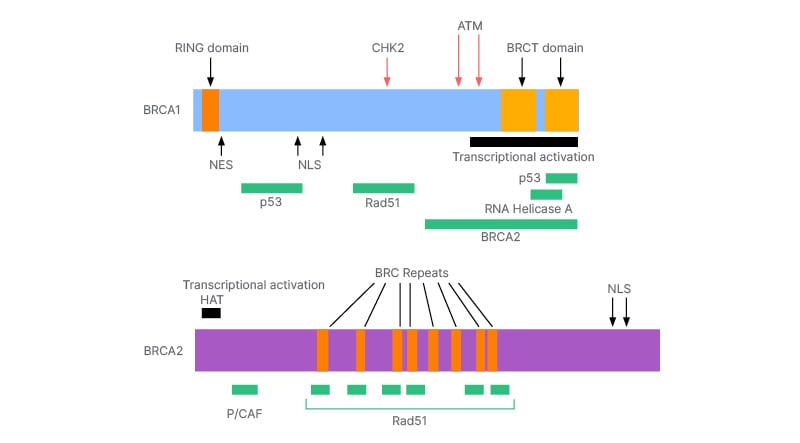
Figure 1: The arrangement of protein on BRCA1 and BRCA2.
The mechanism of action of both BRCA1 and BRCA2 indicate the significance of BRCA in the cell cycle and cell regulation, as these genes encode proteins that function to limit proliferation. However, if the tumour suppressor genes are inactivated by a point mutation, deletion, or loss of expression, there is no longer any restraint on tissue growth. The loss of heterozygosity (LOH) is observed in ovarian cancer in the BRCA1 or BRCA2 allele. Such cases are exquisitely sensitive to DNA damaging agents like platinum and poly(ADP-ribose) polymerase (PARP) inhibitors.7 Inactivation of proteins interacting with BRCA or deletion of a protein on BRCA may substantially lead to development of breast- and ovarian-related malignancy. It is crucial that patients with breast cancer undergo genetic screening to help prevent and manage BRCA-related ovarian cancer.
Ovarian cancer is also histologically classified into five categories.8 Among these, BRCA mutation is mostly seen in high-grade serous carcinoma.
METHODS
The authors conducted an electronic search using various databases, including Medline through Ovid, PubMed, Embase, and Google Scholar. The goal was to find articles related to keywords such as ‘ovarian cancer’, ‘ovarian neoplasm’, ‘ovarian carcinoma’, ‘ovarian malignancies’, and terms like ‘BRCA1’, ‘BRCA2’, or ‘BRCA’, in conjunction with ‘genetic screening’, ‘genetic testing’, and ‘preventive measures’. This search focused solely on studies involving humans, without any language restrictions. Moreover, the authors manually checked reference lists from relevant studies to see how the information applied to their research. To prevent overlap in patient groups, e.g., if authors wrote about the same cohorts in multiple publications, the study authors only included the most recent or detailed study in their analysis. For a study to be eligible for inclusion, it had to meet the following set of criteria: address ovarian cancer, note BRCA mutations, discuss preventive measures, and be published after January 2010.
Data Extraction and Methodological Assessment
The reports the authors retrieved contained information such as authorship, publication year, journal name, sample size, methodologies used, histology types, and preventive strategies employed. To minimise bias during data extraction, three reviewers independently gathered the required data.
RISK ASSESSMENT MODELS
The main goal of genetic testing for harmful mutations in the BRCA1/2 genes is to pinpoint women who face the highest risk of developing ovarian cancer. Doing this means that effective preventive measures can be taken. It is important to note that pathogenic BRCA1/2 mutations are quite rare; they occur in about 1/800–1/1000 individuals for each gene.9 These mutations tend to occur more frequently among individuals or families with certain risk factors, including early onset (<50 years of age), prior cases of breast cancer or ovarian cancer within the family tree, multiple cancers (either similar or related), family history of male breast cancer, or among those at higher risk for founder mutations linked to specific ancestries like Ashkenazi Jewish or Swedish backgrounds.10
Advancements in cancer genetics have raised awareness about tailored risk assessments concerning primary cancer prevention.11 A thorough risk evaluation requires detailed family histories plus comprehensive medical insights from patients. Understanding both clinical prediction models and the underlying pathophysiology and aetiology of breast and ovarian cancer risks are crucial. Given these complexities, professional organisations suggest that only trained healthcare providers in the field of genetics should carry out risk assessments. This way they can accurately counsel patients while minimising potential harms.12,13 Currently utilised models include those provided by the NCCN and ACOG. Furthermore, models like BRCAPRO and BOADICEA have been created to estimate the likelihood that a person might have a pathogenic BRCA1/2 mutation based on their family history.14
GUIDELINE ON GENETIC TESTING
Patients often look into genetic testing either after learning about a mutation from a relative or qualifying through personalised screening following a diagnosis of breast or ovarian cancer at a young age (≤50 years). NCCN guidelines provide clear criteria for referring individuals to genetic specialists based on both personal and family cancer histories 15
SCREENING TOOLS AND ALGORITHM
Currently available guidelines do not recommend routine ultrasound for ovarian cancer screening unless it is before undertaking risk-reducing prophylactic bilateral salpingo-oophorectomy (RRSO) at age 35–40 years or once childbearing is completed.16,17 Still, some carriers of BRCA mutations may opt for screenings so that they can manage their fertility and overall quality of life. Organisations like the NCCN and ACOG now consider image-based screenings reasonable for short-term monitoring in women younger than 35 years until they proceed with prophylactic surgeries.18 Conventional practices for screening high-risk women include using serial serum CA-125 tests paired with annual transvaginal ultrasound featuring Doppler evaluation.19 Research indicates that frequent CA-125 tests offer more value compared to one-time measurements while noting that CA-125 levels can be normal or only slightly elevated during early-stage ovarian cancer.20 That is why relying solely on serum CA-125 measurements is not sufficient for proper screening. The combination approach remains the only evidence-based option currently accessible to at-risk patients; however, better screening methods are still being explored.
COUNSELLING
Genetic counselling for BRCA1/2 mutation testing should only be performed by qualified health professionals, including well-trained primary care providers. The genetic counselling process involves detailed analyses concerning families and assessing the risks associated with potentially harmful mutations. It also focuses on identifying suitable candidates for testing alongside providing education about the process itself. This includes discussing the benefits and drawbacks linked with genetic testing and interpreting results post-testing, as well as reviewing management options moving forward. Pre- and post-test genetic counselling is also supported by the NCCN guidelines.15
PREVENTIVE MEASURES
Increasing incidence of ovarian cancer warrants the implementation of appropriate preventive strategies. This becomes even more important for BRCA mutation carriers who are at an increased risk of ovarian cancer. The current preventive gold standard is RRSO. Another surgical alternative to this is prophylactic salpingectomy with delayed oophorectomy (PSDO).
Chemoprevention, a developing area, deals with the preventive efficacy of certain drugs, including but not limited to oral contraceptive pills. Other prospective candidates include retinoids and other phytochemicals, anti-angiogenic agents, and vitamin D analogues.
Risk-Reducing Salpingo-Oopherectomy
This is a surgical procedure to remove both fallopian tubes and ovaries, which substantially reduces the risk of ovarian and fallopian tube cancer in BRCA mutation carriers.21 The specific protocol for high-risk women involves exploring the pelvic organs for any evidence of cancer, peritoneal wash, and removal of the ovaries and fallopian tubes in their entirety.22 A prospective study of 80 women in China enrolled for RRSO had an overall 4.1% rate of cancer in mutation carriers, out of which 74 had deleterious gene mutations: 58.1% BRCA 1 and 35.1% BRCA2.23 Analysis by Eleje at al.,22 showed that RRSO may improve overall survival and reduce mortality from high-grade serous carcinomas (HGSC) and breast cancer. Overall quality of life is not affected by RRSO, but lower cancer-related anxiety is reported, with most women very satisfied with their choice of risk-reducing surgery.24
A standardised histopathological sectioning and extensively examining the fimbria (SEE-FIM) protocol should be used after RRSO for identifying invasive cancers, which, if detected, should be referred to a tertiary gynaecological oncology centre for appropriate management.25
Though RRSO remains the gold standard for reduction of the risk of ovarian cancer in BRCA carriers, it is associated with significant side-effects. The acute loss of oestrogen exposure induces premature menopause with short-term effects including vasomotor complaints such as hot flushes, sleep disturbances, and impaired sexual functioning, as well as long-term effects, which include a risk of cardiovascular disease, osteoporosis, and cognitive impairment.24,26
Prophylactic Salpingectomy with Delayed Oophorectomy
Even though advanced stages of the disease often appear in the ovaries, sometimes right at the clinical presentation, it has been suggested that the ovarian surface epithelium might not be where it all starts.27
The idea of the fallopian tube or the Müllerian model was initially put forth by Louis Dubaeu.28 Now, it is well known that many ovarian HGSCs likely come from the distal fimbrial end of the fallopian tube. This is linked to a precursor known as serous tubal intraepithelial carcinoma (STIC). In contrast, low-grade serous carcinomas (LGSC) generally arise within the ovary from benign or borderline serous tumours.29 The most common histological subtype is HGSC, more than 95% of which is characterised by mutations in the tumour suppressor gene TP53, known as the p53 signature.30-33
For those with BRCA mutations, if a p53 signature is found in the fallopian tubes, it suggests HGSC may likely come from there.34,35 Consequently, PSDO is being floated as a less morbid alternative to RRSO in BRCA mutation carriers.36-39 This novel strategy has been demonstrated to have positive effects on menopause-related quality of life and sexual health when compared to RRSO.40 This strategy involves two steps.41 First, all salpingeal tissue is surgically removed after childbearing is complete (or sooner if assisted reproductive technology is anticipated), followed by oophorectomy at a later point. When performing salpingectomy, doctors inspect the peritoneum and take peritoneal washings for cytology. They also use SEE-FIM processing on the fallopian tube to check for precursor lesions and sometimes hidden cancers.42
This technique is, however, discouraged in clinical practice outside of clinical trials due to a lack of sufficient evidence on oncological safety. The UK Cancer Genetics Group (CGG) and British Gynaecological Cancer Society (BGCS) pointed out several main concerns: limited data showing benefits (83%), increased surgical risks (79%), loss of breast cancer risk reduction (68%), need for long-term follow-up (61%), and a percentage that may not proceed with the second procedure (66%).43
Oral Contraceptives
Oral contraceptives (OCP) are associated with a significant reduction in risk of ovarian cancer and are an important preventive factor for most histological types.44 A meta-analysis by Iodice et al.,45 provides evidence that in women with an ascertained germ line mutation in BRCA1 or BRCA2, OCPs reduce ovarian cancer risk and found no evidence that formulations after 1975 increased the risk of breast cancer.45 The risk of ovarian cancer was more strongly reduced with longer durations of use, and the relative risks remain low for a long period, attenuating 20 years after stopping.46
The exact mechanism by which OCPs confer long-lasting protection against ovarian cancer is not well understood, but a causal association can be inferred from its ability to suppress ovarian activity.47
Among women who use a combined oestrogen–progesterone therapy, the annual risk of breast cancer increases with the duration of use and dissipates within 2 years of cessation of therapy.48 However, data on breast cancer risk associated with OCP use in BRCA mutation carriers is heterogeneous, and hence, a theoretical risk should be considered when giving clinical recommendations.49
Non-steroidal Anti-inflammatory Drugs
Analysis of observational epidemiological studies suggests an at least 10% relative risk reduction of ovarian cancer with aspirin use.50 Aspirin has been found in vitro to reverse the metabolic derangements such as the increased activation of hexokinase–2 activation, resulting in increased glycolysis caused by loss of BRCA1.51 Animal experiments conducted by Guo et al.,52 concluded that the anti-cancer effect of aspirin is due to increased p53 activation in a concentration-dependent manner, which enhances sensitivity to a combination of cisplatin and aspirin.52
Non-aspirin NSAIDs like acetaminophen and ibuprofen have statistically non-significant relationships with the risk of ovarian cancer.53
Potential gastrointestinal adverse effects and heterogeneity in data, coupled with the absence of data on frequency and dose of NSAIDs and the role of age, discourage regular use of NSAIDs as chemoprotective agents.54,55
Others
Retinoids
Retinoids have a wide range of pleiotropic effects, but monotherapy is not likely to be an effective prevention strategy, and analysis of combination therapies to address toxicity and resistance issues is needed.56 Cytoplasmic retinol binding protein (CRBP)-1, the most diffuse CRBP isoform, is frequently downregulated or lost in ovarian cancer.57,58
Fenretinide is a synthetic analogue of all-trans retinoic acid that was first produced in the late 1960’s.59 It is somewhat protective against ovarian cancers in women with germline BRCA mutations, and further investigation is required.60
Anti-angiogenic agents
Targeting multiple points in the angiogenesis pathway can help in the prevention of cancer in high-risk individuals, especially with BRCA.61 Anti-angiogenic strategies mainly aim to inhibit the early stages of angiogenesis where there are leaky intercellular connections with irregular blood flow.62,63
Wang et al.,64 evaluated 10 phytochemicals and found that oleanolic acid, silibinin, curcumin, epigallocatechin gallate, melatonin, and resveratrol have anti-tumourigenic effects in almost all cancer hallmarks, along with strong anti-tumour/anti-angiogenic action. They suggested further experimental tests for developing these as complements or alternative agents.64
Curcumin
Curcumin has been shown to inhibit the expression of MMP-9, VEGF, and HER2.65,66 The anti-cancer activity is believed to be exerted through inhibiting proliferation and inducing apoptosis in a wide array of cancer cell types in vitro, including ovarian cells.67
Limited bioavailability is a limitation, but its safety profile and nominal cost make it a candidate to explore as an agent for chemoprevention.68
Vitamin D
Meta-analyses recommend a converse relationship between circulating Vitamin D3 levels with rates of ovarian and other cancers.69,70 It has been demonstrated that vitamin D biosynthesis and signalling is compromised in the ovarian epithelium of women with BRCA1 mutation.71
Growing evidence suggests that vitamin D deficiency is correlated to ovarian cancer and that calcitriol and its analogues could be promising agents for cancer prevention.72
The active metabolite of vitamin D, calcitriol, has been found to inhibit proliferation of the ovarian cancer cell line OVCAR4.73







