BACKGROUND
Although immunotherapy is better tolerated globally than chemotherapy, immune checkpoint inhibitors may be associated with immune-related adverse events (irAE), characterised by their diversity, unpredictability, and corticosteroid responsiveness.1,2 The author investigated the ImmunoTOX meeting’s activities: a multidisciplinary real life and case-by-case approach to manage irAE and capture the salient medical needs in the management of immunological toxicities.
METHODS
The ImmunoTOX assessment board is an academic, multidisciplinary group of oncologists and organ specialists that was set up in April 2016 at Gustave Roussy cancer centre in France. The board meets every 2 weeks to discuss the case-by-case management of patients presenting with irAE. This report describes ImmunoTOX’s meeting activities between 6th April 2016 and 2nd January 2019.
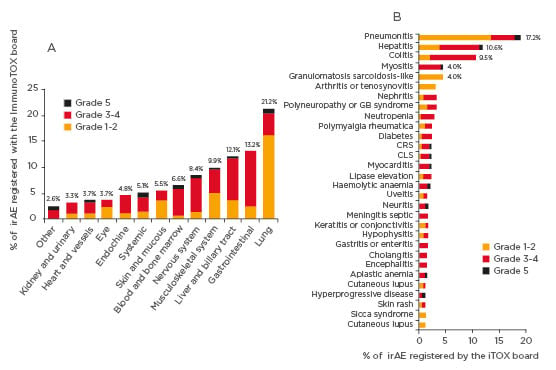
Figure 1: Distribution of irAE organ categories considered by the ImmunoTOX assessment board. In the figure, only irAE that occurred in three or more patients are shown.
CLS: capillary leak system; CRS: cytokine release syndrome; GB syndrome: Guillain-Barré syndrome; irAE: immune-related adverse event.
RESULTS
Over the 32 months of study period, 398 requests concerning 356 patients were submitted to the ImmunoTOX board. The most common tumour types of patients were thoracic cancers (n=105, 29%), skin cancers (n=82, 23%), and renal carcinomas (n=28, 8%). The requests most frequently asked were causal link between immunotherapy and adverse event (n=148, 37%), the possibility for retreatment after hold due to previous adverse event (n=109, 27%), the clinical management of complex situation (n=100, 25%), and the initiation of immunotherapy in patients with pre-existing comorbidities (n=41, 10%). The ImmunoTOX board found a relationship between immunotherapy and adverse event in 273 (77%) of the 356 patients. The organ systems most frequently involved by irAE were the lung (n=58, 21%), gastrointestinal tract (n=36, 13%), liver or biliary tract (n=33, 12%), musculoskeletal system (n=27, 10%), and nervous system (n=23, 8%). The retreatment by immunotherapy after holding due to previous adverse event and the initiation of immunotherapy in patients with pre-existing autoimmune comorbidities were assessed as precaution for use and not formally contraindication in 65% and 93% of the cases, respectively.
CONCLUSION
The medical needs in the management of immune-related adverse events involves five salient organ systems, namely the lung, gastrointestinal, liver and biliary tract, musculoskeletal, and nervous systems. Retreatment after holding due to previous adverse events and immunotherapy initiation in patients with autoimmune comorbidities were mostly assessed as precaution for use and not formal contraindication. A multidisciplinary and case by case approach can be helpful and complementary of general guidelines in the management of immunological toxicities.








